APOE Christchurch-mimetic therapeutic antibody reduces APOE-mediated toxicity and tau phosphorylation
- PMID: 37791598
- PMCID: PMC10916992
- DOI: 10.1002/alz.13436
APOE Christchurch-mimetic therapeutic antibody reduces APOE-mediated toxicity and tau phosphorylation
Abstract
Introduction: We discovered that the APOE3 Christchurch (APOE3Ch) variant may provide resistance to Alzheimer's disease (AD). This resistance may be due to reduced pathological interactions between ApoE3Ch and heparan sulfate proteoglycans (HSPGs).
Methods: We developed and characterized the binding, structure, and preclinical efficacy of novel antibodies targeting human ApoE-HSPG interactions.
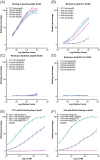
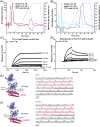
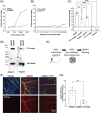
Results: We found that one of these antibodies, called 7C11, preferentially bound ApoE4, a major risk factor for sporadic AD, and disrupts heparin-ApoE4 interactions. We also determined the crystal structure of a Fab fragment of 7C11 and used computer modeling to predict how it would bind to ApoE. When we tested 7C11 in mouse models, we found that it reduced recombinant ApoE-induced tau pathology in the retina of MAPT*P301S mice and curbed pTau S396 phosphorylation in brains of systemically treated APOE4 knock-in mice. Targeting ApoE-HSPG interactions using 7C11 antibody may be a promising approach to developing new therapies for AD.
Keywords: Alzheimer's disease; ApoE; ApoE Christchurch; Apolipoprotein E; HSPG; drug development; heparan sulfate proteoglycan; heparin; tau phosphorylation; tauopathy; therapeutic antibody.
© 2023 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Drs Y. Quiroz, F. Lopera and J. Arboleda‐Velasquez are inventors on a patent filed by Mass General Brigham to leverage therapeutics inspired by the APOE Christchurch findings. Dr Y. Quiroz received grants from the National Institute on Aging, the Alzheimer's Association and Massachusetts General Hospital ECOR. Dr Y. Quiroz is and Editorial Board Member of this journal but was not involved in the peer‐review process nor had access to any information regarding its peer‐review and serves as a consultant for Biogen. Drs. J. Arboleda‐Velasquez and L. A. Kim are co‐founders of Epoch Biotech, an LLC developing resilient case‐inspired therapeutics. F. Lopera received consulting fees from Biogen and Tecnoquimicas. All other authors have no conflicts to disclose. Author disclosures are available in the supporting information.
Figures


References
-
- Karlawish J, Grill JD. The approval of Aduhelm risks eroding public trust in Alzheimer research and the FDA. Nat Rev Neurol. 2021;17(9):523‐524. - PubMed
-
- Tanzi RE. FDA approval of aduhelm paves a new path for Alzheimer's disease. ACS Chem Neurosci. 2021;12(15):2714‐2715. - PubMed
-
- Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature. 2016;537(7618):50‐56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

