Simultaneous Nanorheometry and Nanothermometry Using Intracellular Diamond Quantum Sensors
- PMID: 37791968
- PMCID: PMC10604098
- DOI: 10.1021/acsnano.3c05285
Simultaneous Nanorheometry and Nanothermometry Using Intracellular Diamond Quantum Sensors
Abstract
The viscoelasticity of the cytoplasm plays a critical role in cell morphology, cell division, and intracellular transport. Viscoelasticity is also interconnected with other biophysical properties, such as temperature, which is known to influence cellular bioenergetics. Probing the connections between intracellular temperature and cytoplasmic viscoelasticity provides an exciting opportunity for the study of biological phenomena, such as metabolism and disease progression. The small length scales and transient nature of changes in these parameters combined with their complex interdependencies pose a challenge for biosensing tools, which are often limited to a single readout modality. Here, we present a dual-mode quantum sensor capable of performing simultaneous nanoscale thermometry and rheometry in dynamic cellular environments. We use nitrogen-vacancy centers in diamond nanocrystals as biocompatible sensors for in vitro measurements. We combine subdiffraction resolution single-particle tracking in a fluidic environment with optically detected magnetic resonance spectroscopy to perform simultaneous sensing of viscoelasticity and temperature. We use our sensor to demonstrate probing of the temperature-dependent viscoelasticity in complex media at the nanoscale. We then investigate the interplay between intracellular forces and the cytoplasmic rheology in live cells. Finally, we identify different rheological regimes and reveal evidence of active trafficking and details of the nanoscale viscoelasticity of the cytoplasm.
Keywords: biosensing; nanodiamond; nitrogen-vacancy center; quantum sensing; rheometry; single particle tracking; thermometry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

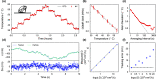
 . (d) Comparison between the known position
(cyan data) in the x-direction of a nanodiamond moved
in a Brownian motion-manner and the tracker-reported position (blue
data), with the corresponding difference (δ x) shown in the lower panel. The set diffusion coefficient is 2 ×
103 nm2/s for this measurement. (e) The measured
diffusion coefficient using the mean square displacement (MSD) at
a time interval of 1 s shows a close agreement with the input diffusion
coefficient. (f) The dynamic tracking accuracy, which is the standard
deviation of the discrepancy between the tracker and particle trajectory,
depends on the diffusion coefficient. When the particle is stationary,
our system has a benchmark spatial resolution of 3.7 nm with 9.6 ms
update rate (black dashed curve).
. (d) Comparison between the known position
(cyan data) in the x-direction of a nanodiamond moved
in a Brownian motion-manner and the tracker-reported position (blue
data), with the corresponding difference (δ x) shown in the lower panel. The set diffusion coefficient is 2 ×
103 nm2/s for this measurement. (e) The measured
diffusion coefficient using the mean square displacement (MSD) at
a time interval of 1 s shows a close agreement with the input diffusion
coefficient. (f) The dynamic tracking accuracy, which is the standard
deviation of the discrepancy between the tracker and particle trajectory,
depends on the diffusion coefficient. When the particle is stationary,
our system has a benchmark spatial resolution of 3.7 nm with 9.6 ms
update rate (black dashed curve).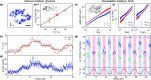

References
-
- Squires T. M.; Mason T. G. Fluid Mechanics of Microrheology. Annu. Rev. Fluid Mech. 2010, 42, 413–438. 10.1146/annurev-fluid-121108-145608. - DOI
-
- Hurst S.; Vos B. E.; Brandt M.; Betz T. Intracellular softening and increased viscoelastic fluidity during division. Nat. Phys. 2021, 17, 1270–1276. 10.1038/s41567-021-01368-z. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

