CRB1-Associated Retinal Dystrophy Patients Have Expanded Lewis Glycoantigen-Positive T Cells
- PMID: 37792335
- PMCID: PMC10565706
- DOI: 10.1167/iovs.64.13.6
CRB1-Associated Retinal Dystrophy Patients Have Expanded Lewis Glycoantigen-Positive T Cells
Abstract
Purpose: Eye inflammation may occur in patients with inherited retinal dystrophies (IRDs) and is seen frequently in IRDs associated with mutations in the CRB1 gene. The purpose of this study was to determine the types of inflammatory cells involved in IRDs, by deep profiling the composition of peripheral blood mononuclear cells of patients with a CRB1-associated IRD.
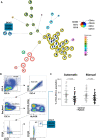
Methods: This study included 33 patients with an IRD with confirmed CRB1 mutations and 32 healthy controls. A 43-parameter flow cytometry analysis was performed on peripheral blood mononuclear cells isolated from venous blood. FlowSOM and manual Boolean combination gating were used to identify and quantify immune cell subsets.
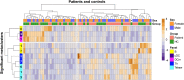
Results: Comparing patients with controls revealed a significant increase in patients in the abundance of circulating CD4+ T cells and CD8+ T cells that express sialyl Lewis X antigen. Furthermore, we detected a decrease in plasmacytoid dendritic cells and an IgA+CD24+CD38+ transitional B-cell subset in patients with an IRD.
Conclusions: Patients with a CRB1-associated IRD show marked changes in blood leukocyte composition, affecting lymphocyte and dendritic cell populations. These results implicate inflammatory pathways in the disease manifestations of IRDs.
Conflict of interest statement
Disclosure:
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

