Dynamic extracellular vestibule of human SERT: Unveiling druggable potential with high-affinity allosteric inhibitors
- PMID: 37792512
- PMCID: PMC10576121
- DOI: 10.1073/pnas.2304089120
Dynamic extracellular vestibule of human SERT: Unveiling druggable potential with high-affinity allosteric inhibitors
Abstract
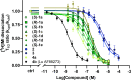
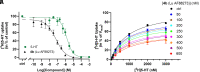
The serotonin transporter (SERT) tightly regulates synaptic serotonin levels and has been the primary target of antidepressants. Binding of inhibitors to the allosteric site of human SERT (hSERT) impedes the dissociation of antidepressants bound at the central site and may enhance the efficacy of such antidepressants to potentially reduce their dosage and side effects. Here, we report the identification of a series of high-affinity allosteric inhibitors of hSERT in a unique scaffold, with the lead compound, Lu AF88273 (3-(1-(2-(1H-indol-3-yl)ethyl)piperidin-4-yl)-6-chloro-1H-indole), having 2.1 nM allosteric potency in inhibiting imipramine dissociation. In addition, we find that Lu AF88273 also inhibits serotonin transport in a noncompetitive manner. The binding pose of Lu AF88273 in the allosteric site of hSERT is determined with extensive molecular dynamics simulations and rigorous absolute binding free energy perturbation (FEP) calculations, which show that a part of the compound occupies a dynamically formed small cavity. The predicted binding location and pose are validated by site-directed mutagenesis and can explain much of the structure-activity relationship of these inhibitors using the relative binding FEP calculations. Together, our findings provide a promising lead compound and the structural basis for the development of allosteric drugs targeting hSERT. Further, they demonstrate that the divergent allosteric sites of neurotransmitter transporters can be selectively targeted.
Keywords: allosteric binding site; allosteric modulator; free energy perturbation; molecular dynamics; serotonin transporter.
Conflict of interest statement
The authors declare no competing interest.
Figures
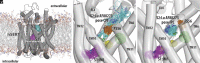
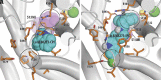

References
-
- Zhdanava M., et al. , The prevalence and national burden of treatment-resistant depression and major depressive disorder in the United States. J. Clin. Psychiatry 82, 20m13699 (2021). - PubMed
-
- Kristensen A. S., et al. , SLC6 neurotransmitter transporters: Structure, function, and regulation. Pharmacol. Rev. 63, 585–640 (2011). - PubMed
-
- Rudnick G., Talvenheimo J., Fishkes H., Nelson P. J., Sodium ion requirements for serotonin transport and imipramine binding. Psychopharmacol. Bull. 19, 545–549 (1983). - PubMed
-
- Nelson P. J., Rudnick G., Coupling between platelet 5-hydroxytryptamine and potassium transport. J. Biol. Chem. 254, 10084–10089 (1979). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

