Triple-Negative Breast Cancer: Histopathologic Features, Genomics, and Treatment
- PMID: 37792593
- PMCID: PMC10560981
- DOI: 10.1148/rg.230034
Triple-Negative Breast Cancer: Histopathologic Features, Genomics, and Treatment
Abstract
Triple-negative breast cancer (TNBC) is a heterogeneous and aggressive group of tumors that are defined by the absence of estrogen and progesterone receptors and lack of ERBB2 (formerly HER2 or HER2/neu) overexpression. TNBC accounts for 8%-13% of breast cancers. In addition, it accounts for a higher proportion of breast cancers in younger women compared with those in older women, and it disproportionately affects non-Hispanic Black women. TNBC has high metastatic potential, and the risk of recurrence is highest during the 5 years after it is diagnosed. TNBC exhibits benign morphologic imaging features more frequently than do other breast cancer subtypes. Mammography can be suboptimal for early detection of TNBC owing to factors that include the fast growth of this cancer, increased mammographic density in young women, and lack of the typical features of malignancy at imaging. US is superior to mammography for TNBC detection, but benign-appearing features can lead to misdiagnosis. Breast MRI is the most sensitive modality for TNBC detection. Most cases of TNBC are treated with neoadjuvant chemotherapy, followed by surgery and radiation. MRI is the modality of choice for evaluating the response to neoadjuvant chemotherapy. Survival rates for individuals with TNBC are lower than those for persons with hormone receptor-positive and human epidermal growth factor receptor 2-positive cancers. The 5-year survival rates for patients with localized, regional, and distant disease at diagnosis are 91.3%, 65.8%, and 12.0%, respectively. The early success of immunotherapy has raised hope regarding the development of personalized strategies to treat TNBC. Imaging and tumor biomarkers are likely to play a crucial role in the prediction of TNBC treatment response and TNBC patient survival in the future. ©RSNA, 2023 Quiz questions for this article are available in the supplemental material.
Conflict of interest statement
Figures


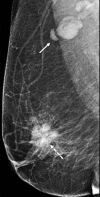
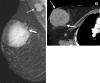
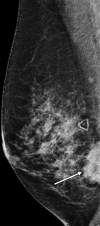

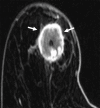
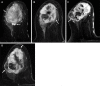
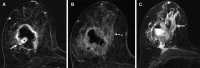
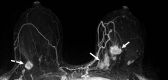
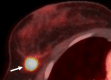
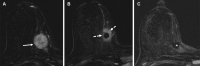
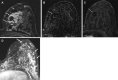
![Left breast palpable abnormality in a 39-year-old woman. (A) Left
craniocaudal mammogram shows a 0.9-cm oval mass (dashed arrow) in the upper
outer breast correlating with the palpable area of concern (triangular
marker [*] ). This lesion was shown to represent an intramammary
lymph node at subsequent US. A 1-cm oval mass (solid arrow) at the posterior
depth was incidentally noted. (B) On a transverse US image, the 1-cm mass
appears to have an oval shape and circumscribed margins (arrow). (C)
Six-month follow-up US image shows a 1.4-cm irregularly shaped mass with
microlobulated margins (arrow). US-guided biopsy revealed TNBC.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/6dfb/10560981/e968b82ad0b0/rg.230034.fig8.gif)
References
-
- Centers for Disease Control and Prevention . Breast Cancer Statistics . Centers for Disease Control and Prevention website https://www.cdc.gov/cancer/breast/statistics/index.htm. Updated June 6, 2022. Accessed February 1, 2023 .
-
- Trop I , LeBlanc SM , David J , et al. . Molecular classification of infiltrating breast cancer: toward personalized therapy . RadioGraphics 2014. ; 34 ( 5 ): 1178 – 1195 . - PubMed
-
- National Cancer Institute . Cancer Stat Facts: Female Breast Cancer Subtypes . National Institutes of Health, National Cancer Institute website https://seer.cancer.gov/statfacts/html/breast-subtypes.html. Accessed March 28, 2023 .
-
- Howard FM , Olopade OI . Epidemiology of triple-negative breast cancer: a review . Cancer J 2021. ; 27 ( 1 ): 8 – 16 . - PubMed
-
- Liedtke C , Mazouni C , Hess KR , et al. . Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer . J Clin Oncol 2008. ; 26 ( 8 ): 1275 – 1281 . [Published correction appears in J Clin Oncol 2023;41(10):1809-1815.] - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

