Stem-Cell Aging and Pathways to Precancer Evolution
- PMID: 37792614
- PMCID: PMC11908798
- DOI: 10.1056/NEJMra2304431
Stem-Cell Aging and Pathways to Precancer Evolution
Figures
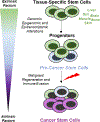

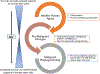

References
-
- Till J, McCulloch E. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res 1961;14:213–22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
