Target trial emulation: Do antimicrobials or gastrointestinal nutraceuticals prescribed at first presentation for acute diarrhoea cause a better clinical outcome in dogs under primary veterinary care in the UK?
- PMID: 37792702
- PMCID: PMC10550114
- DOI: 10.1371/journal.pone.0291057
Target trial emulation: Do antimicrobials or gastrointestinal nutraceuticals prescribed at first presentation for acute diarrhoea cause a better clinical outcome in dogs under primary veterinary care in the UK?
Abstract
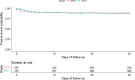
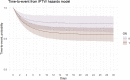
Target trial emulation applies design principles from randomised controlled trials to the analysis of observational data for causal inference and is increasingly used within human epidemiology. Veterinary electronic clinical records represent a potentially valuable source of information to estimate real-world causal effects for companion animal species. This study employed the target trial framework to evaluate the usefulness on veterinary observational data. Acute diarrhoea in dogs was used as a clinical exemplar. Inclusion required dogs aged ≥ 3 months and < 10 years, presenting for veterinary primary care with acute diarrhoea during 2019. Treatment strategies were: 1. antimicrobial prescription compared to no antimicrobial prescription and 2. gastrointestinal nutraceutical prescription compared to no gastrointestinal nutraceutical prescription. The primary outcome was clinical resolution (defined as no revisit with ongoing diarrhoea within 30 days from the date of first presentation). Informed from a directed acyclic graph, data on the following covariates were collected: age, breed, bodyweight, insurance status, comorbidities, vomiting, reduced appetite, haematochezia, pyrexia, duration, additional treatment prescription and veterinary group. Inverse probability of treatment weighting was used to balance covariates between the treatment groups for each of the two target trials. The risk difference (RD) of 0.4% (95% CI -4.5% to 5.3%) was non-significant for clinical resolution in dogs treated with antimicrobials compared with dogs not treated with antimicrobials. The risk difference (RD) of 0.3% (95% CI -4.5% to 5.0%) was non-significant for clinical resolution in dogs treated with gastrointestinal nutraceuticals compared with dogs not treated with gastrointestinal nutraceuticals. This study successfully applied the target trial framework to veterinary observational data. The findings show that antimicrobial or gastrointestinal prescription at first presentation of acute diarrhoea in dogs causes no difference in clinical resolution. The findings support the recommendation for veterinary professionals to limit antimicrobial use for acute diarrhoea in dogs.
Copyright: © 2023 Pegram et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Frey BB. The SAGE Encyclopedia of Educational Research, Measurement, and Evaluation. 2018.
-
- Maringe C, Benitez Majano S, Exarchakou A, Smith M, Rachet B, Belot A, et al.. Reflection on modern methods: trial emulation in the presence of immortal-time bias. Assessing the benefit of major surgery for elderly lung cancer patients using observational data. International Journal of Epidemiology. 2020;49(5):1719–29. doi: 10.1093/ije/dyaa057 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

