Moderate intensity continuous versus high intensity interval training: Metabolic responses of slow and fast skeletal muscles in rat
- PMID: 37792807
- PMCID: PMC10550171
- DOI: 10.1371/journal.pone.0292225
Moderate intensity continuous versus high intensity interval training: Metabolic responses of slow and fast skeletal muscles in rat
Abstract
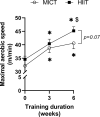
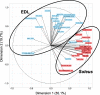
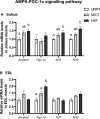
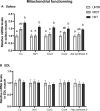
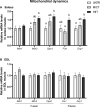
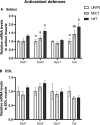
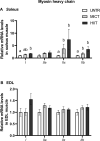
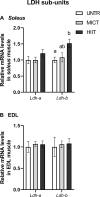
The healthy benefits of regular physical exercise are mainly mediated by the stimulation of oxidative and antioxidant capacities in skeletal muscle. Our understanding of the cellular and molecular responses involved in these processes remain often uncomplete particularly regarding muscle typology. The main aim of the present study was to compare the effects of two types of exercise training protocol: a moderate-intensity continuous training (MICT) and a high-intensity interval training (HIIT) on metabolic processes in two muscles with different typologies: soleus and extensor digitorum longus (EDL). Training effects in male Wistar rats were studied from whole organism level (maximal aerobic speed, morphometric and systemic parameters) to muscle level (transcripts, protein contents and enzymatic activities involved in antioxidant defences, aerobic and anaerobic metabolisms). Wistar rats were randomly divided into three groups: untrained (UNTR), n = 7; MICT, n = 8; and HIIT, n = 8. Rats of the MICT and HIIT groups ran five times a week for six weeks at moderate and high intensity, respectively. HIIT improved more than MICT the endurance performance (a trend to increased maximal aerobic speed, p = 0.07) and oxidative capacities in both muscles, as determined through protein and transcript assays (AMPK-PGC-1α signalling pathway, antioxidant defences, mitochondrial functioning and dynamics). Whatever the training protocol, the genes involved in these processes were largely more significantly upregulated in soleus (slow-twitch fibres) than in EDL (fast-twitch fibres). Solely on the basis of the transcript changes, we conclude that the training protocols tested here lead to specific muscular responses.
Copyright: © 2023 Pengam et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

