Allogeneic hematopoietic cell transplantation for blastic plasmacytoid dendritic cell neoplasm: a CIBMTR analysis
- PMID: 37792849
- PMCID: PMC10690553
- DOI: 10.1182/bloodadvances.2023011308
Allogeneic hematopoietic cell transplantation for blastic plasmacytoid dendritic cell neoplasm: a CIBMTR analysis
Abstract
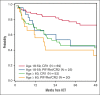
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare hematological malignancy with a poor prognosis and considered incurable with conventional chemotherapy. Small observational studies reported allogeneic hematopoietic cell transplantation (allo-HCT) offers durable remissions in patients with BPDCN. We report an analysis of patients with BPDCN who received an allo-HCT, using data reported to the Center for International Blood and Marrow Transplant Research (CIBMTR). We identified 164 patients with BPDCN from 78 centers who underwent allo-HCT between 2007 and 2018. The 5-year overall survival (OS), disease-free survival (DFS), relapse, and nonrelapse mortality (NRM) rates were 51.2% (95% confidence interval [CI], 42.5-59.8), 44.4% (95% CI, 36.2-52.8), 32.2% (95% CI, 24.7-40.3), and 23.3% (95% CI, 16.9-30.4), respectively. Disease relapse was the most common cause of death. On multivariate analyses, age of ≥60 years was predictive for inferior OS (hazard ratio [HR], 2.16; 95% CI, 1.35-3.46; P = .001), and higher NRM (HR, 2.19; 95% CI, 1.13-4.22; P = .02). Remission status at time of allo-HCT (CR2/primary induction failure/relapse vs CR1) was predictive of inferior OS (HR, 1.87; 95% CI, 1.14-3.06; P = .01) and DFS (HR, 1.75; 95% CI, 1.11-2.76; P = .02). Use of myeloablative conditioning with total body irradiation (MAC-TBI) was predictive of improved DFS and reduced relapse risk. Allo-HCT is effective in providing durable remissions and long-term survival in BPDCN. Younger age and allo-HCT in CR1 predicted for improved survival, whereas MAC-TBI predicted for less relapse and improved DFS. Novel strategies incorporating allo-HCT are needed to further improve outcomes.
Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: H.S.M. reports advisory board participation from CRISPR Therapeutics, Senti Biosciences, Jazz Pharmaceuticals, and Incyte Corporation. S.A. reports compensation (research funding) from Seattle Genetics, Merck, Xencor, and Tessa Therapeutics, and has a membership on Tessa Therapeutic’s advisory committee. S.G. reports advisory board participation for Bristol Myers Squibb, Sanofi, Astellas, Daiichi Sankyo, Kite Pharma, Janssen, and AstraZeneca, and speaker’s bureau participation for Seattle Genetics. T.N. reports clinical trial support (institution) from Novartis and clinical trial support (drug only supply to the institution) from Karyopharm. M.B. reports research support to institution from Novartis. M.R.G. reports receiving consulting fees from AbbVie, Amgen, Astellas, Blueprint Medicines, Bristol Myers Squibb, Cardinal Health, CTI BioPharma, Daiichi Sankyo, Gamida Cell, Genentech, Gilead, GlaxoSmithKline/Sierra Oncology, Incyte, Invitae, Jazz, Karius, Novartis, Ono Pharmaceutical, Pfizer, Pharmacosmos, Premier, Servier/Agios, and Stemline Therapeutics; research support from Incyte and Janssen; and stock ownership for Medtronic. N.K. reports honorarium from Optum for educational event. H.L. reports advisory board meeting participation for Agios, Pfizer, Nkarta, CTI Biopharm, and BeiGene; research support from Bristol Myers Squibb, Karyopharm, and Miltenyi Biotec; being a consultant for NGM BioPharma; and being a speaker/lecturer for Society of Immunotherapy of Cancer and Chinese American Hematologist and Oncologist Network. M.U. reports advisory board participation for Stemline and Gilead. N.B. reports advisory board participation or consultancy services for Magenta Therapeutics, Medexus Pharma, CTI BioPharma, CareDx and Sanofi. V.R.B. reports safety monitoring committee participation for Protagonist; consulting fees from Genentech, Rigel, Agios, Incyte, Servier Pharmaceuticals LLC, Omeros, Takeda, Partnership for Health Analytic Research, LLC (which in turn, receives funds from Jazz Pharmaceuticals), and AbbVie; research funding (institutional) from AbbVie, Pfizer, Incyte, Jazz, Tolero Pharmaceuticals, Inc, and National Marrow Donor Program; and drug support (institutional) from Oncoceutics for a trial. S.C. reports research funding (institutional) from Bristol Myers Squibb, Amgen, Janssen, Novartis, Syndax, Ionis, Sanofi, and GlaxoSmithKline, and honorarium from GlaxoSmithKline, Sanofi (advisory board), and Omeros (speaker’s bureau). E.C. reports consultative council participation for Amgen. B.D. reports research funding (institutional) from Janssen, Angiocrine, Pfizer, Poseida, MEI, and Orcabio, and consultancy/adviser for Jazz, Gamida Cell, MJH BioScience, Arivan Research, and BEAM Therapeutics. J.J. reports advisory board meeting participation for Elzonris in January 2022. M.M.K. reports being a consultant for Secura Bio (unrelated to current study). N.P. reports consultancy for AbbVie, Genentech, Takeda, and Foundation One, and research support from AbbVie, Genentech, and Incyte. D.M. reports advisory board participation for MorphoSys and Seagen; research funding from Genentech, MorphoSys, and ADC Therapeutics; and honorarium from AstraZeneca. P.N.M. reports being a consultant for Kite and speaker’s bureau participation for Incyte. A.M. reports an investigator grant from Gilead. D.A.R. reports advisory board and speaker’s bureau participation for Stemline Therapeutics. A.S. reports being a consultant for Spotlight Therapeutics in 2020, Medexus Inc in 2021, and Vertex Pharmaceuticals in 2021 and 2022; research funding from CRISPR Therapeutics in 2021 and 2022; and research collaboration with Magenta Therapeutics since 2021. C.U. reports honoraria for advisory board or speaker’s bureau participation from Blueprint and Takeda (unrelated to current study). P.K. reports compensation (advisory board participation) for Jazz, Kite, and Pfizer, and clinical trial support from Amgen and Ziopharm. C.S.H. reports a government conflict of interest. The remaining authors declare no competing financial interests.
Figures
References
-
- Pagano L, Valentini CG, Grammatico S, Pulsoni A. Blastic plasmacytoid dendritic cell neoplasm: diagnostic criteria and therapeutical approaches. Br J Haematol. 2016;174(2):188–202. - PubMed
-
- Brody JP, Allen S, Schulman P, et al. Acute agranular CD4-positive natural killer cell leukemia. Comprehensive clinicopathologic studies including virologic and in vitro culture with inducing agents. Cancer. 1995;75(10):2474–2483. - PubMed
-
- DiGiuseppe JA, Louie DC, Williams JE, et al. Blastic natural killer cell leukemia/lymphoma: a clinicopathologic study. Am J Surg Pathol. 1997;21(10):1223–1230. - PubMed