Metal-Catalyzed Enantioconvergent Transformations
- PMID: 37793021
- PMCID: PMC10603790
- DOI: 10.1021/acs.chemrev.3c00059
Metal-Catalyzed Enantioconvergent Transformations
Abstract
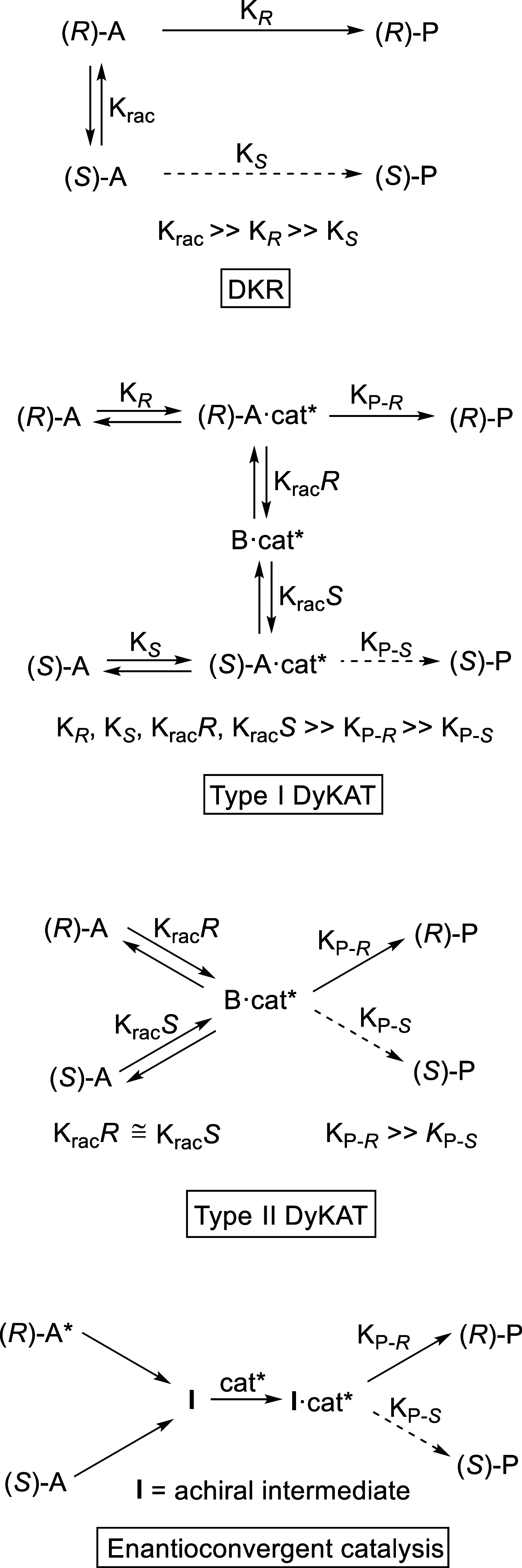
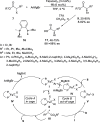
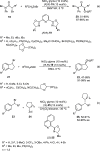
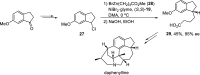
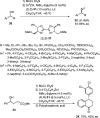
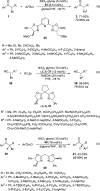
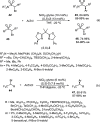
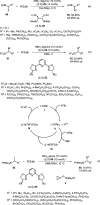
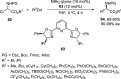
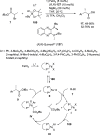
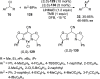
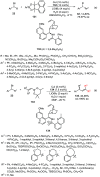
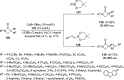
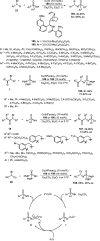
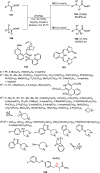
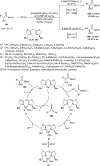
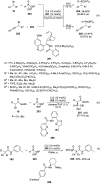
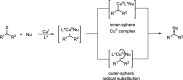
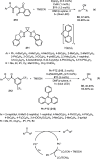
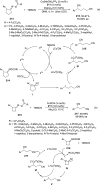
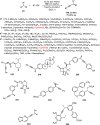
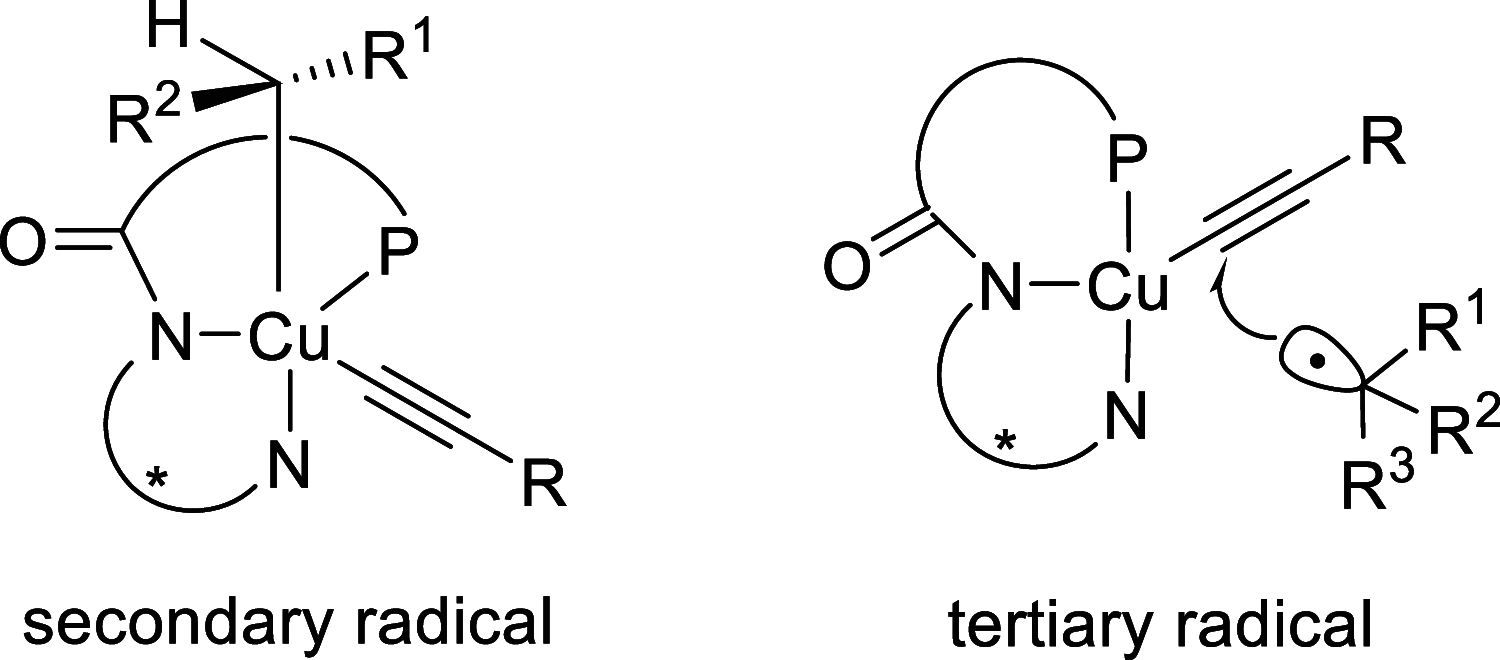
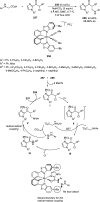
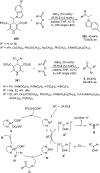
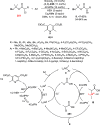
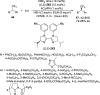
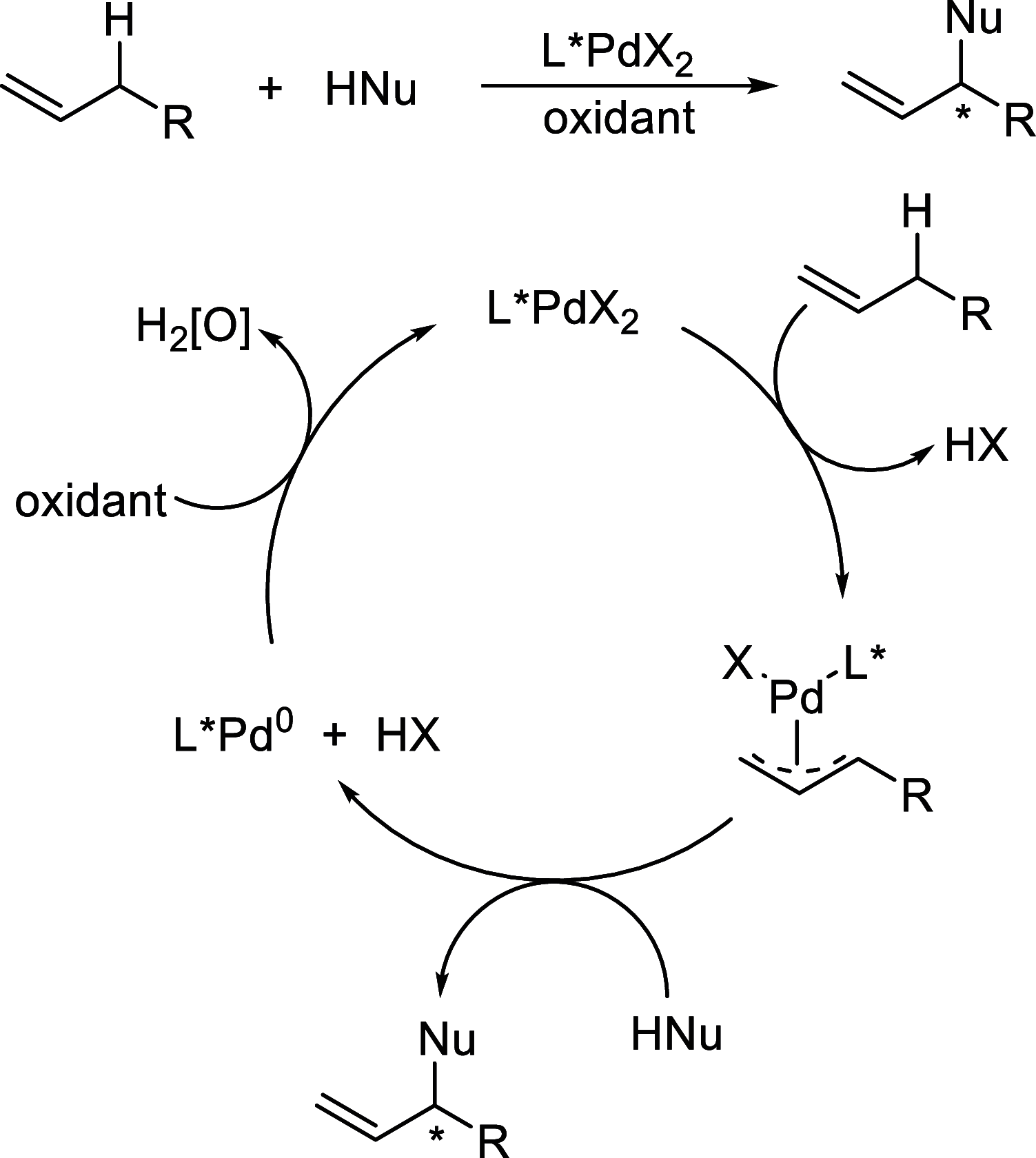
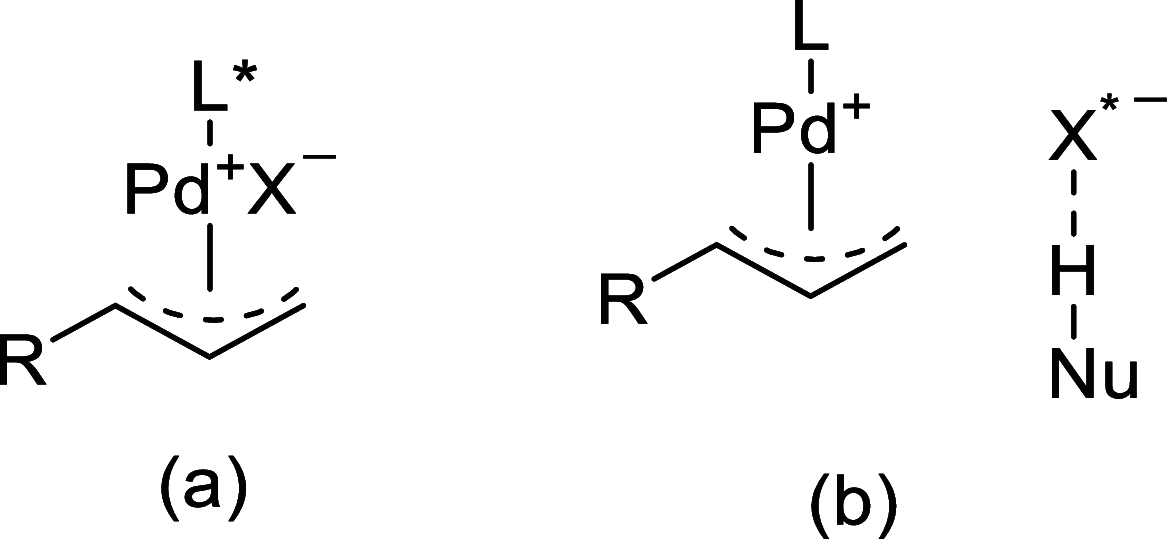
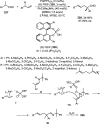
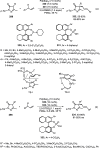
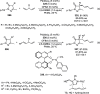
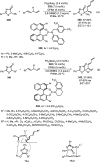
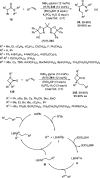
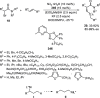
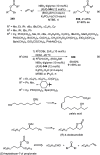
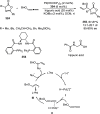
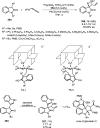
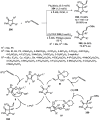
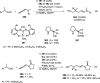
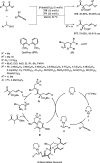
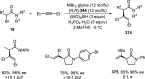
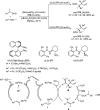
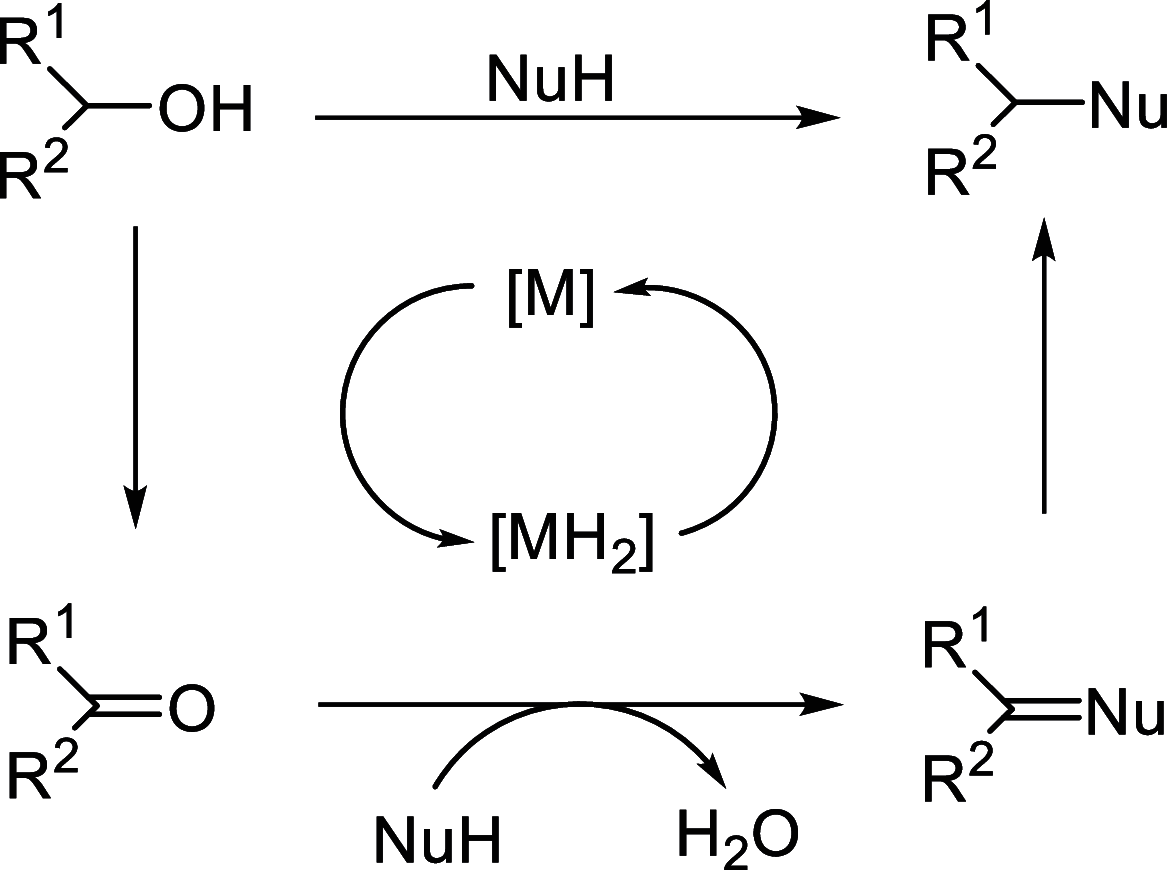
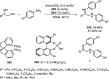
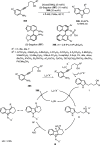
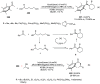
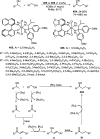
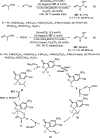
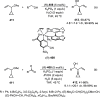
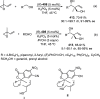
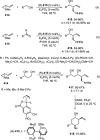
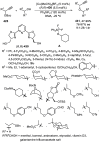
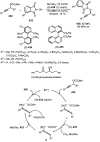
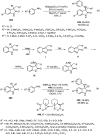
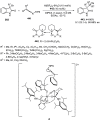
Enantioconvergent catalysis has expanded asymmetric synthesis to new methodologies able to convert racemic compounds into a single enantiomer. This review covers recent advances in transition-metal-catalyzed transformations, such as radical-based cross-coupling of racemic alkyl electrophiles with nucleophiles or racemic alkylmetals with electrophiles and reductive cross-coupling of two electrophiles mainly under Ni/bis(oxazoline) catalysis. C-H functionalization of racemic electrophiles or nucleophiles can be performed in an enantioconvergent manner. Hydroalkylation of alkenes, allenes, and acetylenes is an alternative to cross-coupling reactions. Hydrogen autotransfer has been applied to amination of racemic alcohols and C-C bond forming reactions (Guerbet reaction). Other metal-catalyzed reactions involve addition of racemic allylic systems to carbonyl compounds, propargylation of alcohols and phenols, amination of racemic 3-bromooxindoles, allenylation of carbonyl compounds with racemic allenolates or propargyl bromides, and hydroxylation of racemic 1,3-dicarbonyl compounds.
Conflict of interest statement
The authors declare no competing financial interest.
Figures















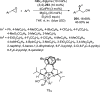

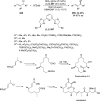
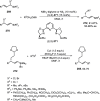
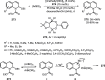
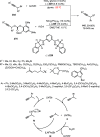
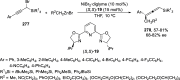
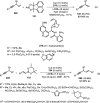
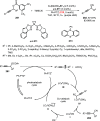

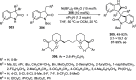
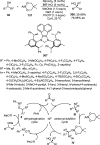
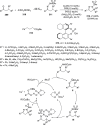
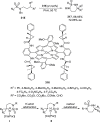


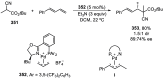
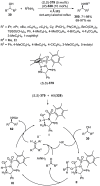
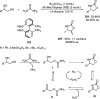
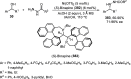
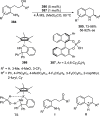




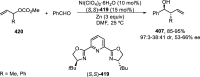
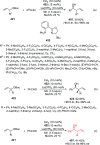
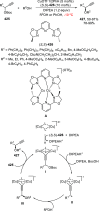
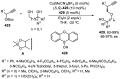

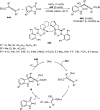
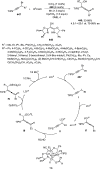
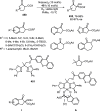
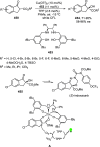
References
-
- Willis M. C. Enantioselective Desymmetrization. J. Chem. Soc., Perkin Trans. 1 1999, 1765–1784. 10.1039/a906269b. - DOI
-
- Wang M.; Feng M.; Tang B.; Jiang X. Recent Advances of Desymmetrization Protocol Applied in Natural Product Total Synthesis. Tetrahedron Lett. 2014, 55, 7147–7155. 10.1016/j.tetlet.2014.10.152. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

