MyPathway Human Epidermal Growth Factor Receptor 2 Basket Study: Pertuzumab + Trastuzumab Treatment of a Tissue-Agnostic Cohort of Patients With Human Epidermal Growth Factor Receptor 2-Altered Advanced Solid Tumors
- PMID: 37793085
- PMCID: PMC10824375
- DOI: 10.1200/JCO.22.02636
MyPathway Human Epidermal Growth Factor Receptor 2 Basket Study: Pertuzumab + Trastuzumab Treatment of a Tissue-Agnostic Cohort of Patients With Human Epidermal Growth Factor Receptor 2-Altered Advanced Solid Tumors
Abstract
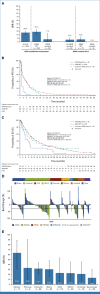
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.The MyPathway multiple-basket study (ClinicalTrials.gov identifier: NCT02091141) is evaluating targeted therapies in nonindicated tumors with relevant molecular alterations. We assessed pertuzumab + trastuzumab in a tissue-agnostic cohort of adult patients with human epidermal growth factor receptor 2 (HER2)-amplified and/or -overexpressed and/or -mutated solid tumors. The primary end point was objective response rate (ORR); secondary end points included survival and safety. At data cutoff (March 2022), 346 patients with HER2 amplification and/or overexpression with/without HER2 mutations (n = 263), or HER2 mutations alone (n = 83) had been treated. Patients with HER2 amplification and/or overexpression had an ORR of 25.9% (68/263, 95% CI, 20.7 to 31.6), including five complete responses (urothelial [n = 2], salivary gland [n = 2], and colon [n = 1] cancers). Activity was higher in those with wild-type (ORR, 28.1%) versus mutated KRAS (ORR, 7.1%). Among patients with HER2 amplification, ORR was numerically higher in patients with immunohistochemistry (IHC) 3+ (41.0%; 32/78) or 2+ (21.9%; 7/32), versus 1+ (8.3%; 1/12) or no expression (0%; 0/20). In patients with HER2 mutations alone, ORR was 6.0% (5/83, 95% CI, 2.0 to 13.5). Pertuzumab + trastuzumab showed activity in various HER2-amplified and/or -overexpressed tumors with wild-type KRAS, with the range of activity dependent on tumor type, but had limited activity in the context of KRAS mutations, HER2 mutations alone, or 0-1+ HER2 expression.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
References
-
- Dumbrava EEI, Balaji K, Raghav K, et al. : Targeting ERBB2 (HER2) amplification identified by next-generation sequencing in patients with advanced or metastatic solid tumors beyond conventional indications. JCO Precis Oncol 10.1200/PO.18.00345 - DOI - PMC - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, et al. : Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783-792, 2001 - PubMed
-
- Bang YJ, Van Cutsem E, Feyereislova A, et al. : Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 376:687-697, 2010 - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

