Neutrophils resist ferroptosis and promote breast cancer metastasis through aconitate decarboxylase 1
- PMID: 37793345
- PMCID: PMC10558089
- DOI: 10.1016/j.cmet.2023.09.004
Neutrophils resist ferroptosis and promote breast cancer metastasis through aconitate decarboxylase 1
Abstract
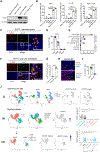
Metastasis causes breast cancer-related mortality. Tumor-infiltrating neutrophils (TINs) inflict immunosuppression and promote metastasis. Therapeutic debilitation of TINs may enhance immunotherapy, yet it remains a challenge to identify therapeutic targets highly expressed and functionally essential in TINs but under-expressed in extra-tumoral neutrophils. Here, using single-cell RNA sequencing to compare TINs and circulating neutrophils in murine mammary tumor models, we identified aconitate decarboxylase 1 (Acod1) as the most upregulated metabolic enzyme in mouse TINs and validated high Acod1 expression in human TINs. Activated through the GM-CSF-JAK/STAT5-C/EBPβ pathway, Acod1 produces itaconate, which mediates Nrf2-dependent defense against ferroptosis and upholds the persistence of TINs. Acod1 ablation abates TIN infiltration, constrains metastasis (but not primary tumors), bolsters antitumor T cell immunity, and boosts the efficacy of immune checkpoint blockade. Our findings reveal how TINs escape from ferroptosis through the Acod1-dependent immunometabolism switch and establish Acod1 as a target to offset immunosuppression and improve immunotherapy against metastasis.
Keywords: Acod1; MDSC; breast cancer; ferroptosis; immune checkpoint blockade; immune metabolism; itaconate; metastasis; neutrophil; single-cell RNA sequencing.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

