Comparison of sequential versus concurrent chemoradiation regimens in non-metastatic muscle-invasive bladder cancer
- PMID: 37793624
- PMCID: PMC10556844
- DOI: 10.3857/roj.2023.00262
Comparison of sequential versus concurrent chemoradiation regimens in non-metastatic muscle-invasive bladder cancer
Abstract
Purpose: The treatment approach for non-metastatic bladder cancer is guided by an invasion of the muscular layer of the bladder wall. Radical cystectomy is the recommended treatment for muscle-invasive disease. However, it has considerable morbidity and mortality and is not suited for many patients. Trimodality therapy consisting of chemoradiation after transurethral resection of bladder tumor offers a definitive approach with bladder-sparing potential. However, there is a lack of research defining the optimal combination of chemotherapy and radiation in this setting.
Materials and methods: We extracted patient data from the National Cancer Database to compare survival outcomes and demographic factors in 2,227 non-metastatic bladder cancer patients who were treated with chemotherapy sequential to or concurrently with radiation. Sequential treatment was defined as chemotherapy beginning >14 days before radiation, and concurrent was defined as beginning within 14 days of the first radiation.
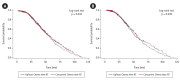
Results: The sequential treatment group patients were younger (mean age, 74 vs. 78 years; p < 0.001) with more advanced disease. We found no difference in overall survival between patients who received chemotherapy sequential to radiation and those who received concurrent chemoradiation only (p = 0.533).
Conclusion: Our data are concordant with a previous prospective study, and support that chemotherapy prior to radiation does not decrease survival outcomes relative to patients receiving only concurrent chemoradiation. Given that the sequential group had an overall higher stage but no difference in survival, downstaging chemotherapy prior to radiation may be helpful in these patients. Further studies including a larger, multi-institutional clinical trial are indicated to support clinical decision-making.
Keywords: Chemoradiotherapy; Survival analysis; Urinary bladder neoplasms.
Conflict of interest statement
Benjamin A. Teply reports funding for clinical research from Bristol-Myers-Squibb, and Advisory Board Consultancy for Seagen and Astra Zeneca. Raymond C. Bergan is on the Scientific advisory Committee for the National Cancer Institute, Northwestern University, and University of Arizona. All other authors have no conflicts of interest to declare.
Figures

References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. - PubMed
-
- National Cancer Institute, Surveillance, Epidemiology, and End Results Program . Bethesda, MD: National Cancer Institute; c2023. Cancer Stat Facts: bladder cancer [Internet] [cited 2023 Aug 10]. Available from: https://seer.cancer.gov/statfacts/html/urinb.html.
-
- Flaig TW, Spiess PE, Agarwal N, et al. Bladder cancer, version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2020;18:329–54. - PubMed
-
- Rodel C, Grabenbauer GG, Kuhn R, et al. Combined-modality treatment and selective organ preservation in invasive bladder cancer: long-term results. J Clin Oncol. 2002;20:3061–71. - PubMed
-
- Efstathiou JA, Spiegel DY, Shipley WU, et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: the MGH experience. Eur Urol. 2012;61:705–11. - PubMed
LinkOut - more resources
Full Text Sources

