SGN-B7H4V, an investigational vedotin ADC directed to the immune checkpoint ligand B7-H4, shows promising activity in preclinical models
- PMID: 37793853
- PMCID: PMC10551938
- DOI: 10.1136/jitc-2023-007572
SGN-B7H4V, an investigational vedotin ADC directed to the immune checkpoint ligand B7-H4, shows promising activity in preclinical models
Abstract
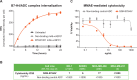
Background: SGN-B7H4V is a novel investigational vedotin antibody-drug conjugate (ADC) comprising a B7-H4-directed human monoclonal antibody conjugated to the cytotoxic payload monomethyl auristatin E (MMAE) via a protease-cleavable maleimidocaproyl valine citrulline (mc-vc) linker. This vedotin linker-payload system has been clinically validated in multiple Food and Drug Administration approved agents including brentuximab vedotin, enfortumab vedotin, and tisotumab vedotin. B7-H4 is an immune checkpoint ligand with elevated expression on a variety of solid tumors, including breast, ovarian, and endometrial tumors, and limited normal tissue expression. SGN-B7H4V is designed to induce direct cytotoxicity against target cells by binding to B7-H4 on the surface of target cells and releasing the cytotoxic payload MMAE upon internalization of the B7-H4/ADC complex.
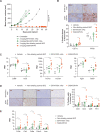
Methods: B7-H4 expression was characterized by immunohistochemistry across multiple solid tumor types. The ability of SGN-B7H4V to kill B7-H4-expressing tumor cells in vitro and in vivo in a variety of xenograft tumor models was also evaluated. Finally, the antitumor activity of SGN-B7H4V as monotherapy and in combination with an anti-programmed cell death-1 (PD-1) agent was evaluated using an immunocompetent murine B7-H4-expressing Renca tumor model.
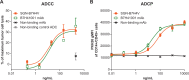
Results: Immunohistochemistry confirmed B7-H4 expression across multiple solid tumors, with the highest prevalence in breast, endometrial, and ovarian tumors. In vitro, SGN-B7H4V killed B7-H4-expressing tumor cells by MMAE-mediated direct cytotoxicity and antibody-mediated effector functions including antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis. In vivo, SGN-B7H4V demonstrated strong antitumor activity in multiple xenograft models of breast and ovarian cancer, including xenograft tumors with heterogeneous B7-H4 expression, consistent with the ability of vedotin ADCs to elicit a bystander effect. In an immunocompetent murine B7-H4-expressing tumor model, SGN-B7H4V drove robust antitumor activity as a monotherapy that was enhanced when combined with an anti-PD-1 agent.
Conclusion: The immune checkpoint ligand B7-H4 is a promising molecular target expressed by multiple solid tumors. SGN-B7H4V demonstrates robust antitumor activity in preclinical models through multiple potential mechanisms. Altogether, these preclinical data support the evaluation of SGN-B7H4V as a monotherapy in the ongoing phase 1 study of SGN-B7H4V in advanced solid tumors (NCT05194072) and potential future clinical combinations with immunotherapies.
Keywords: Drug Evaluation, Preclinical; Drug Therapy, Combination; Therapies, Investigational; Translational Medical Research; Tumor Microenvironment.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: EG, MU, AE, PY, DS, KH, SA, L-YH, JH, KG, PMT, EST, JJG, RT, SW, KS, EJH, KSn, DM, MP, AJS, CF, JPS, NN, and SJG were employees of and had equity ownership in Seagen Inc. at the time of this work.
Figures



References
-
- ADCETRIS . Brentuximab Vedotin (ADCETRIS). FDA Prescribing Information,
-
- PADCEV . Enfortumab Vedotin (PADCEV). FDA Prescribing Information,
-
- Tivdak . Tisotumab vedotin (Tivdak). FDA Prescribing Information,
-
- ClinicalTrials.gov . A study of SGN-B7H4V in advanced solid tumors. 2022. Available: https://www.clinicaltrials.gov/ct2/show/NCT05194072 [Accessed 14 Apr 2023].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
