A thermostable type I-B CRISPR-Cas system for orthogonal and multiplexed genetic engineering
- PMID: 37794017
- PMCID: PMC10551041
- DOI: 10.1038/s41467-023-41973-5
A thermostable type I-B CRISPR-Cas system for orthogonal and multiplexed genetic engineering
Abstract
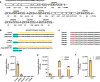
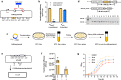
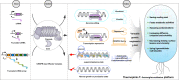
Thermophilic cell factories have remarkably broad potential for industrial applications, but are limited by a lack of genetic manipulation tools and recalcitrance to transformation. Here, we identify a thermophilic type I-B CRISPR-Cas system from Parageobacillus thermoglucosidasius and find it displays highly efficient transcriptional repression or DNA cleavage activity that can be switched by adjusting crRNA length to less than or greater than 26 bp, respectively, without ablating Cas3 nuclease. We then develop an orthogonal tool for genome editing and transcriptional repression using this type I-B system in both thermophile and mesophile hosts. Empowered by this tool, we design a strategy to screen the genome-scale targets involved in transformation efficiency and established dynamically controlled supercompetent P. thermoglucosidasius cells with high efficiency ( ~ 108 CFU/μg DNA) by temporal multiplexed repression. We also demonstrate the construction of thermophilic riboflavin cell factory with hitherto highest titers in high temperature fermentation by genome-scale identification and combinatorial manipulation of multiple targets. This work enables diverse high-efficiency genetic manipulation in P. thermoglucosidasius and facilitates the engineering of thermophilic cell factories.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors have filed a provisional patent for this work to the China National Intellectual Property Administration (CNIPA: 202310705228X). W.W., Z.Y., Z.L., and R.B. are inventors on the provisional patent application. The remaining authors declare no competing interests.
Figures




References
-
- Ro DK, et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440:940–943. - PubMed
-
- Wang W, et al. Harnessing the intracellular triacylglycerols for titer improvement of polyketides in Streptomyces. Nat. Biotechnol. 2020;38:76–83. - PubMed
-
- Chen GQ, Jiang XR. Next generation industrial biotechnology based on extremophilic bacteria. Curr. Opin. Biotechnol. 2018;50:94–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

