Intravenous administration of BCG in mice promotes natural killer and T cell-mediated antitumor immunity in the lung
- PMID: 37794033
- PMCID: PMC10551006
- DOI: 10.1038/s41467-023-41768-8
Intravenous administration of BCG in mice promotes natural killer and T cell-mediated antitumor immunity in the lung
Abstract
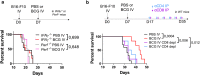
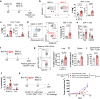
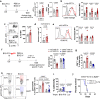
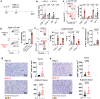
Intravesical administration of Bacillus Calmette-Guérin (BCG) was one of the first FDA-approved immunotherapies and remains a standard treatment for bladder cancer. Previous studies have demonstrated that intravenous (IV) administration of BCG is well-tolerated and effective in preventing tuberculosis infection in animals. Here, we examine IV BCG in several preclinical lung tumor models. Our findings demonstrate that BCG inoculation reduced tumor growth and prolonged mouse survival in models of lung melanoma metastasis and orthotopic lung adenocarcinoma. Moreover, IV BCG treatment was well-tolerated with no apparent signs of acute toxicity. Mechanistically, IV BCG induced tumor-specific CD8+ T cell responses, which were dependent on type 1 conventional dendritic cells, as well as NK cell-mediated immunity. Lastly, we also show that IV BCG has an additive effect on anti-PD-L1 checkpoint inhibitor treatment in mouse lung tumors that are otherwise resistant to anti-PD-L1 as monotherapy. Overall, our study demonstrates the potential of systemic IV BCG administration in the treatment of lung tumors, highlighting its ability to enhance immune responses and augment immune checkpoint blockade efficacy.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

