Genotype-stratified treatment for monogenic insulin resistance: a systematic review
- PMID: 37794082
- PMCID: PMC10550936
- DOI: 10.1038/s43856-023-00368-9
Genotype-stratified treatment for monogenic insulin resistance: a systematic review
Erratum in
-
Author Correction: Genotype-stratified treatment for monogenic insulin resistance: a systematic review.Commun Med (Lond). 2024 Mar 26;4(1):57. doi: 10.1038/s43856-024-00482-2. Commun Med (Lond). 2024. PMID: 38532029 Free PMC article. No abstract available.
Abstract
Background: Monogenic insulin resistance (IR) includes lipodystrophy and disorders of insulin signalling. We sought to assess the effects of interventions in monogenic IR, stratified by genetic aetiology.
Methods: Systematic review using PubMed, MEDLINE and Embase (1 January 1987 to 23 June 2021). Studies reporting individual-level effects of pharmacologic and/or surgical interventions in monogenic IR were eligible. Individual data were extracted and duplicates were removed. Outcomes were analysed for each gene and intervention, and in aggregate for partial, generalised and all lipodystrophy.
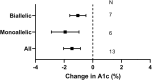
Results: 10 non-randomised experimental studies, 8 case series, and 23 case reports meet inclusion criteria, all rated as having moderate or serious risk of bias. Metreleptin use is associated with the lowering of triglycerides and haemoglobin A1c (HbA1c) in all lipodystrophy (n = 111), partial (n = 71) and generalised lipodystrophy (n = 41), and in LMNA, PPARG, AGPAT2 or BSCL2 subgroups (n = 72,13,21 and 21 respectively). Body Mass Index (BMI) is lowered in partial and generalised lipodystrophy, and in LMNA or BSCL2, but not PPARG or AGPAT2 subgroups. Thiazolidinediones are associated with improved HbA1c and triglycerides in all lipodystrophy (n = 13), improved HbA1c in PPARG (n = 5), and improved triglycerides in LMNA (n = 7). In INSR-related IR, rhIGF-1, alone or with IGFBP3, is associated with improved HbA1c (n = 17). The small size or absence of other genotype-treatment combinations preclude firm conclusions.
Conclusions: The evidence guiding genotype-specific treatment of monogenic IR is of low to very low quality. Metreleptin and Thiazolidinediones appear to improve metabolic markers in lipodystrophy, and rhIGF-1 appears to lower HbA1c in INSR-related IR. For other interventions, there is insufficient evidence to assess efficacy and risks in aggregated lipodystrophy or genetic subgroups.
Plain language summary
The hormone insulin stimulates nutrient uptake from the bloodstream into tissues. In insulin resistance (IR), this action is blunted. Some rare gene alterations cause severe IR, diabetes that is difficult to control, and early complications. Many treatments have been suggested, but reliable evidence of their risks and benefits is sparse. We analysed all available reports describing treatment outcomes in severe IR. We found that the evidence is of low to very low quality overall. Injections of leptin, a hormone from fat tissue, or thiazolidinedione tablets that increase fat tissue both appear to improve diabetes control in people with reduced ability to make fat tissue. Injections of another treatment, insulin-like growth factor, appear to improve diabetes control in people with direct blockage of insulin action. There is a pressing need to improve evidence for treatment in these rare and severe conditions.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare the following competing interests: R.K.S. has received speaker fees from Eli Lilly, Novo Nordisk, and Amryt. R. J. B. has received research support from Amryt, Third Rock Ventures, Ionis, and Regeneron. K.A.P. and S.A. report no conflicts of interest.
Figures



Update of
-
Systematic review of genotype-stratified treatment for monogenic insulin resistance.medRxiv [Preprint]. 2023 Apr 21:2023.04.17.23288671. doi: 10.1101/2023.04.17.23288671. medRxiv. 2023. Update in: Commun Med (Lond). 2023 Oct 5;3(1):134. doi: 10.1038/s43856-023-00368-9. PMID: 37205502 Free PMC article. Updated. Preprint.
References
-
- Lim K, Haider A, Adams C, Sleigh A, Savage DB. Lipodistrophy: a paradigm for understanding the consequences of “overloading” adipose tissue. Physiol. Rev. 2021;101:907–993. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

