European LeukemiaNet laboratory recommendations for the diagnosis and management of chronic myeloid leukemia
- PMID: 37794101
- PMCID: PMC10624636
- DOI: 10.1038/s41375-023-02048-y
European LeukemiaNet laboratory recommendations for the diagnosis and management of chronic myeloid leukemia
Abstract
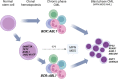
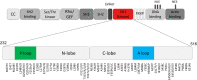
From the laboratory perspective, effective management of patients with chronic myeloid leukemia (CML) requires accurate diagnosis, assessment of prognostic markers, sequential assessment of levels of residual disease and investigation of possible reasons for resistance, relapse or progression. Our scientific and clinical knowledge underpinning these requirements continues to evolve, as do laboratory methods and technologies. The European LeukemiaNet convened an expert panel to critically consider the current status of genetic laboratory approaches to help diagnose and manage CML patients. Our recommendations focus on current best practice and highlight the strengths and pitfalls of commonly used laboratory tests.
© 2023. The Author(s).
Conflict of interest statement
NCPC, TE, KMP, AH and SS and received support from Novartis through the European Treatment and Outcome Study (EUTOS) for CML. NCPC has received research support and honoraria from Novartis, and honoraria from Incyte and Astellas. TE has received research support from BMS, Incyte, Novartis and Pfizer. SB is an advisory board member of Qiagen, Novartis, Terns and Cepheid, received honoraria from Qiagen, Novartis and Cepheid, and research funding from Novartis, Incyte and Cepheid. JMC has received honoraria from Novartis, Incyte, Qiagen and Cepheid. MD has served as a consultant for Ariad, Blueprint Medicine, Bristol Myers Squibb, CTI Biopharma, Cogent, Galena Biopharma, Incyte, Novartis, Pfizer and received research funding from Pfizer. DK served as a member of advisory board for Novartis and Paladin, and received honoraria and research grants from Novartis, Pfizer and Paladin. KMP has received honoraria from Angelini. JR has received honoraria from Novartis, Bristol, Myers Squibb, Takeda, Amgen, Cepheid and Genentech. AH has received institutional research support from Novartis, Bristol Myers Squibb, Incyte, and Pfizer and personal fees from Novartis and Incyte. JFA has received honoraria from Incyte and Novartis and research funding from Incyte and Pfizer. SS has received honoraria from Novartis and Incyte Biosciences. The remaining co-authors report no relevant disclosures.
Figures





References
-
- Nowell PC, Hungerford DA. A Minute Chromosome in Human Chronic Granulocytic Leukemia. Science. 1960;132:1497.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

