Utility and precision evidence of technology in the treatment of type 1 diabetes: a systematic review
- PMID: 37794113
- PMCID: PMC10550996
- DOI: 10.1038/s43856-023-00358-x
Utility and precision evidence of technology in the treatment of type 1 diabetes: a systematic review
Abstract
Background: The greatest change in the treatment of people living with type 1 diabetes in the last decade has been the explosion of technology assisting in all aspects of diabetes therapy, from glucose monitoring to insulin delivery and decision making. As such, the aim of our systematic review was to assess the utility of these technologies as well as identify any precision medicine-directed findings to personalize care.
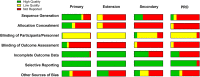
Methods: Screening of 835 peer-reviewed articles was followed by systematic review of 70 of them (focusing on randomized trials and extension studies with ≥50 participants from the past 10 years).
Results: We find that novel technologies, ranging from continuous glucose monitoring systems, insulin pumps and decision support tools to the most advanced hybrid closed loop systems, improve important measures like HbA1c, time in range, and glycemic variability, while reducing hypoglycemia risk. Several studies included person-reported outcomes, allowing assessment of the burden or benefit of the technology in the lives of those with type 1 diabetes, demonstrating positive results or, at a minimum, no increase in self-care burden compared with standard care. Important limitations of the trials to date are their small size, the scarcity of pre-planned or powered analyses in sub-populations such as children, racial/ethnic minorities, people with advanced complications, and variations in baseline glycemic levels. In addition, confounders including education with device initiation, concomitant behavioral modifications, and frequent contact with the healthcare team are rarely described in enough detail to assess their impact.
Conclusions: Our review highlights the potential of technology in the treatment of people living with type 1 diabetes and provides suggestions for optimization of outcomes and areas of further study for precision medicine-directed technology use in type 1 diabetes.
Plain language summary
In the last decade, there have been significant advances in how technology is used in the treatment of people living with type 1 diabetes. These technologies primarily aim to help manage blood sugar levels. Here, we reviewed research published over the last decade to evaluate the impact of such technologies on type 1 diabetes treatment. We find that various types of novel technologies, such as devices to monitor blood sugar levels continuously or deliver insulin, improve important diabetes-related measures and can reduce the risk of having low blood sugar levels. Importantly, several studies showed a positive impact of technologies on quality of life in people living with diabetes. Our findings highlight the benefits of novel technologies in the treatment of type 1 diabetes and identify areas for further research to optimize and personalize diabetes care.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare the following competing interests: J.L.S. serves, or has served, on advisory panels for Bigfoot Biomedical, Cecelia Health, Insulet Corporation, Medtronic Diabetes, StartUp Health Diabetes Moonshot, and Vertex. J.L.S. has served as a consultant to Abbott Diabetes, Bigfoot Biomedical, Insulet, Medtronic Diabetes, and Zealand. Yale School of Medicine has received research support for J.L.S. from Abbott Diabetes, JAEB Center for Health Research, JDRF, Insulet, Medtronic, NIH, and Provention Bio. K.K.H. received consulting fees from Cecelia Health. P.A.G. consults or has consulted for Provention Bio and Viacyte. P.A.G. is the co-founder of IM Therapeutics and serves as CMO on the Board as well as being a shareholder. P.A.G. has also received research support from the Helmsley Foundation, Nova Pharmaceuticals, Imcyse, Provention Bio, Intrexon T1D Partners, and Novartis. All payments have been made to the University of Colorado. P.G. serves, or has served, on the advisory panel for Novo Nordisk, Sanofi-Aventis, Boehringer-Ingelheim, Janssen Pharmaceuticals, Roche, Medtronic, Abbott, Ypsomed, and Bayer. P.G. serves, or has served, on the speaker’s bureau for Merck Sharp and Dohme, Boehringer-Ingelheim, Bayer, Medtronic, Insulet, Novo Nordisk, Abbott, Roche, VitalAire, and Dexcom. Financial compensation for these activities has been received by KU Leuven. KU Leuven received non-financial support for PG for travel from Sanofi-Aventis, A Menarini Diagnostics, Novo Nordisk, Medtronic, and Roche. All disclosures are unrelated to the present work. IBH has received research funding from Insulet and Dexcom and is a consultant for Abbott, embecta, Lifescan, and Hagar. REP reports consulting fees from Bayer AG, Corcept Therapeutics Incorporated, Dexcom, Hanmi Pharmaceutical Co., Merck, Novo Nordisk, Pfizer, Sanofi, Scohia Pharma Inc., and Sun Pharmaceutical Industries, and grants/research support from Hanmi Pharmaceutical Co., Janssen, Metavention, Novo Nordisk, Poxel SA, and Sanofi. All funds are paid directly to REP’s employer, AdventHealth, a nonprofit organization that supports education and research. L.M.L. serves as a consultant for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Janssen, Dexcom, Insulet, Roche, Medtronic, Dompe, Provention Bio, and Vertex. CM serves, or has served, on the advisory panel for Novo Nordisk, Sanofi, Merck Sharp and Dohme Ltd., Eli Lilly and Company, Novartis, AstraZeneca, Boehringer Ingelheim, Roche, Medtronic, ActoBio Therapeutics, Pfizer, Imcyse, Insulet, Zealand Pharma, Avotres and Vertex. Financial compensation for these activities has been received by KU Leuven; KU Leuven has received research support for CM from Medtronic, Imcyse, Novo Nordisk, Sanofi, and ActoBio Therapeutics. L.M.J., E.C., A.C., S.M.P., M.H., S.C., M.U., S.A., M.T., M.J.R., and J.J.W. report no dualities of interest.
Figures



References
-
- Sperling MA, Laffel LM. Current management of glycemia in children with type 1 diabetes mellitus. N. Engl. J. Med. 2022;386:1155–1164. - PubMed
-
- Perkins BA, Sherr JL, Mathieu C. Type 1 diabetes glycemic management: Insulin therapy, glucose monitoring, and automation. Science. 2021;373:522–527. - PubMed
-
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. Clinical Trial Multicenter Study Randomized Controlled Trial Research Support, U.S. Gov’t, P.H.S. N. Engl. J. Med. 1993;329:977–986. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

