The PTPN2/PTPN1 inhibitor ABBV-CLS-484 unleashes potent anti-tumour immunity
- PMID: 37794185
- PMCID: PMC10599993
- DOI: 10.1038/s41586-023-06575-7
The PTPN2/PTPN1 inhibitor ABBV-CLS-484 unleashes potent anti-tumour immunity
Abstract
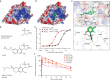
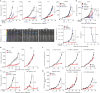
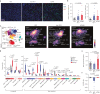
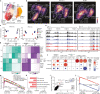
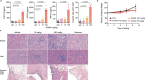
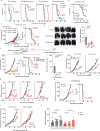
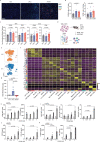
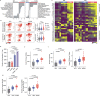
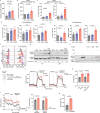
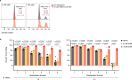
Immune checkpoint blockade is effective for some patients with cancer, but most are refractory to current immunotherapies and new approaches are needed to overcome resistance1,2. The protein tyrosine phosphatases PTPN2 and PTPN1 are central regulators of inflammation, and their genetic deletion in either tumour cells or immune cells promotes anti-tumour immunity3-6. However, phosphatases are challenging drug targets; in particular, the active site has been considered undruggable. Here we present the discovery and characterization of ABBV-CLS-484 (AC484), a first-in-class, orally bioavailable, potent PTPN2 and PTPN1 active-site inhibitor. AC484 treatment in vitro amplifies the response to interferon and promotes the activation and function of several immune cell subsets. In mouse models of cancer resistant to PD-1 blockade, AC484 monotherapy generates potent anti-tumour immunity. We show that AC484 inflames the tumour microenvironment and promotes natural killer cell and CD8+ T cell function by enhancing JAK-STAT signalling and reducing T cell dysfunction. Inhibitors of PTPN2 and PTPN1 offer a promising new strategy for cancer immunotherapy and are currently being evaluated in patients with advanced solid tumours (ClinicalTrials.gov identifier NCT04777994 ). More broadly, our study shows that small-molecule inhibitors of key intracellular immune regulators can achieve efficacy comparable to or exceeding that of antibody-based immune checkpoint blockade in preclinical models. Finally, to our knowledge, AC484 represents the first active-site phosphatase inhibitor to enter clinical evaluation for cancer immunotherapy and may pave the way for additional therapeutics that target this important class of enzymes.
© 2023. The Author(s).
Conflict of interest statement
This manuscript stems from a collaboration between the Broad Institute, AbbVie and Calico Sciences. Equal financial support for the design and study conduct for this research were provided by AbbVie and Calico. R.T.M. and K.B.Y. receive research funding from Calico Life Sciences. C.K.B., K.M.H., K.A.M., K.H., K.L.K., R.D., L.M., R.M., C. Backus, D.E.K., A.C., J.T.K., C.L.H., C.W.H., J.P.D., M.B., J.H.D., Y.Y., M.G., S.F., P. Trusk, W.Q., Q.S., E.P.F., M.A.M., J.M.F. and P.R.K. are employees of AbbVie. P.G. was an employee of AbbVie at the time she contributed to the manuscript and is now an employee of Bristol Myers Squibb. Z.X. was an employee of AbbVie at the time he contributed to the manuscript and is now an employee of Ipsen Therapeutics. K.L. was an employee of AbbVie at the time he contributed to the manuscript. N.K. was an employee of AbbVie at the time he contributed to the manuscript and is now an employee of Pfizer. G.T.H. is a deceased author who was an employee of AbbVie at the time of the study. A.I.-V. was an employee of the Broad Institute at the time he contributed to the manuscript and is now an employee of Monte Rosa Therapeutics. M.N.P., C.H.P., I.-M.S. and Y.L. are employees of Calico Life Sciences. T.R.G. has paid scientific advisory roles and equity in Dewpoint Therapeutics and Anji Pharmaceuticals, holds founder’s equity in Sherlock Biosciences, is a paid advisor to Braidwell and has research funding from Bayer HealthCare, Calico Life Sciences and Novo Holdings. R.T.M. has received consulting or speaking fees from Bristol Myers Squibb, Gilead Sciences and Immunai Therapeutics, has equity ownership in OncoRev, and receives research funding from Calico Life Sciences. C. Beauregard was an employee of Calico Life Sciences at the time he contributed to the manuscript and is now an employee of Vir Biotechnology. AbbVie, Calico Life Sciences and the Broad Institute participated in the interpretation of data, review and approval of the publication. The remaining authors declare no competing interests.
Figures





Comment in
-
Phosphatase inhibitor drives anticancer immune responses.Nat Rev Drug Discov. 2023 Dec;22(12):951. doi: 10.1038/d41573-023-00178-7. Nat Rev Drug Discov. 2023. PMID: 37914810 No abstract available.
-
Small molecule. Big biology. Dual phosphatase inhibitor enters the immunotherapy fray.Immunol Cell Biol. 2024 Jan;102(1):8-11. doi: 10.1111/imcb.12711. Epub 2023 Nov 19. Immunol Cell Biol. 2024. PMID: 37982351
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

