Non-nutritive sweetened beverages versus water after a 52-week weight management programme: a randomised controlled trial
- PMID: 37794246
- PMCID: PMC10746539
- DOI: 10.1038/s41366-023-01393-3
Non-nutritive sweetened beverages versus water after a 52-week weight management programme: a randomised controlled trial
Abstract
Background/objective: Sugar-sweetened beverages are a substantial source of dietary sugar that can contribute to weight gain and the risk of type 2 diabetes. Dietary guidelines recommend non-nutritive sweetened (NNS) beverages to reduce sugar consumption, however, there is a need for long-term randomised controlled trials on their use. We aimed to compare the effects of NNS beverages and water on body weight during weight loss and maintenance in a behavioural weight management programme.
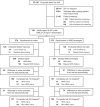
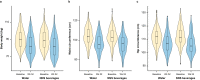
Methods: In this parallel-group, open-label, controlled equivalence trial, adults with a BMI of 27-35 kg/m2 who regularly consumed cold beverages were randomised 1:1 to water or NNS beverages. Participants underwent a group behavioural weight management programme comprising weekly (during the 12-week weight-loss phase) then monthly (during the 40-week weight-maintenance phase) meetings. The primary endpoint was weight change at week 52 (equivalence: two-sided P > 0.05). Secondary endpoints included changes in anthropometrics, cardiometabolic risk factors, appetite and activity levels.
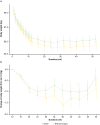
Results: Of 493 participants randomised (water: n = 246; NNS beverages: n = 247), 24.1% were NNS-naïve. At week 52, water and NNS beverages were non-equivalent, with significantly greater weight loss in the NNS beverages group. Participants consuming water maintained a weight loss of 6.1 kg over 52 weeks versus 7.5 kg with NNS beverages (difference [90% CI]: 1.4 kg [-2.6, -0.2]; p < 0.05).
Conclusions: During a 52-week behavioural weight management programme, water and NNS beverages were non-equivalent, with weight loss maintained to a statistically greater extent with NNS beverages compared with water. However, this difference was not clinically significant.
Clinical trial registration: This trial is registered with ClinicalTrials.gov: NCT02591134.
© 2023. The Author(s).
Conflict of interest statement
JAH reports funding (paid to the University of Liverpool) from the American Beverage Association for the present study. Outside of the submitted work, she reports a grant from the European Commission for the Horizon 2020 project SWEET. SH reports funding (paid to the University of Liverpool) from the American Beverage Association for the present study. CR reports funding (paid to the University of Liverpool) from the American Beverage Association for the present study. PThomas reports funding (paid to the University of Liverpool) from the American Beverage Association for the present study. PThorp reports funding (paid to the University of Liverpool) from the American Beverage Association for the present study. CAH reports funding (paid to the University of Liverpool) from the American Beverage Association for the present study. Outside of the submitted work, she reports honoraria from the International Sweeteners Association and International Food Information Council (paid to the University of Liverpool); grants from the European Commission for the Horizon 2020 project SWEET, the Biotechnology & Biological Sciences Research Council and the Natural Environment Research Council; personal fees for her role on the Food Standards Agency Advisory Committee on Social Sciences; and an unpaid role as a trustee of Feeding Liverpool. PC reports funding (paid to the University of Liverpool) from the American Beverage Association for the present study. Outside of the submitted work, he reports grants from the European Commission for the Horizon 2020 project SWEET, the National Institute for Health and Care Research, the Home Office UK and the Federal Bureau of Investigation; and personal fees from Kaplan. JCGH reports funding (paid to the University of Liverpool) from the American Beverage Association for the present study. Outside of the submitted work, he reports a grant from the European Commission for the Horizon 2020 project SWEET; consultancy fees from Boehringer Ingelheim, Dupont and Mars (all paid to the University of Leeds); honoraria from Novo Nordisk (paid to the University of Leeds); support for attending meetings from Novo Nordisk; financial support for participation in an advisory board for Dupont (paid to the University of Leeds); unpaid participation in an advisory board for the Sweet Tooth Project; and unpaid board membership of the European Coalition of People Living with Obesity. He is the President of the European Association for the Study of Obesity.
Figures
References
-
- Public Health England. The Eatwell Guide booklet. 2018. https://www.gov.uk/government/publications/the-eatwell-guide. Accessed 23 Apr 2023.
-
- U.S. Department of Agriculture, U.S. Department of Health and Human Services. Dietary guidelines for Americans, 2020–2025. 2020. https://www.dietaryguidelines.gov/. Accessed 23 Apr 2023.

