Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine
- PMID: 37794253
- PMCID: PMC10735053
- DOI: 10.1038/s41591-023-02502-5
Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine
Abstract
Precision medicine is part of the logical evolution of contemporary evidence-based medicine that seeks to reduce errors and optimize outcomes when making medical decisions and health recommendations. Diabetes affects hundreds of millions of people worldwide, many of whom will develop life-threatening complications and die prematurely. Precision medicine can potentially address this enormous problem by accounting for heterogeneity in the etiology, clinical presentation and pathogenesis of common forms of diabetes and risks of complications. This second international consensus report on precision diabetes medicine summarizes the findings from a systematic evidence review across the key pillars of precision medicine (prevention, diagnosis, treatment, prognosis) in four recognized forms of diabetes (monogenic, gestational, type 1, type 2). These reviews address key questions about the translation of precision medicine research into practice. Although not complete, owing to the vast literature on this topic, they revealed opportunities for the immediate or near-term clinical implementation of precision diabetes medicine; furthermore, we expose important gaps in knowledge, focusing on the need to obtain new clinically relevant evidence. Gaps include the need for common standards for clinical readiness, including consideration of cost-effectiveness, health equity, predictive accuracy, liability and accessibility. Key milestones are outlined for the broad clinical implementation of precision diabetes medicine.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
The following authors report unrelated disclosures. J. Merino. is an Associate Editor of Diabetologia; S.J.C. reports a close family member employed by a Johnson & Johnson company; A.R.K. is supported by the National Center for Advancing Translational Sciences, NIH, through grant KL2TR002490. A.R.K. also reports receiving research grants from the Diabetes Research Connection and the American Diabetes Association, and a research prize from the National Academy of Medicine, outside the submitted work; J.L.T.K. declares lecture fees from Novo Nordisk, international conference costs covered by Medtronic, AstraZeneca and Novo Nordisk; L.-L.L. reports receiving research grants via her academic institution and serving on an advisory board for Boehringer Ingelheim, Novartis, Novo Nordisk, Viatris Pharmaceutical and Zuellig Pharma. L.-L.L. has received speaking honoraria from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Roche, Sanofi, Servier, Viatris Pharmaceutical and Zuellig Pharma for scientific talks over which she had full control of the content; R.M. has received consulting or speaker honoraria from Eli Lilly, Novo Nordisk and Boeringer Engelheim; M.L.M. has received lecture, consultancy, or advisory board fees from Amarin, Amgen, Eli Lilly, Merck Sharp & Dohme, Mylan, Novo Nordisk, Novartis, Servier and SlaPharma; C.E.-M. reports serving on advisory boards for Provention Bio, Isla Technologies, MaiCell Technologies, Avotres, DiogenyX and Neurodon; receiving in-kind research support from Bristol Myers Squibb and Nimbus Pharmaceuticals; receiving investigator-initiated grants from Lilly Pharmaceuticals and Astellas Pharmaceuticals; and having patent (16/291,668) Extracellular Vesicle Ribonucleic Acid (RNA) Cargo as a Biomarker of Hyperglycaemia and Type 1 Diabetes and provisional patent (63/285,765) Biomarker for Type 1 Diabetes (PDIA1 as a biomarker of β cell stress); S.E.G. has been on advisory boards for Abata, Avotres, Genentech, GentiBio, Provention Bio, Sana Biotechnology, Sanofi and is a DSMB member for INNODIA (MELD, ATG), Diamyd (DIAGNODE-3, GAD) and JDRF (TADPOL, DFMO); M.O.G. has served on an advisory board for Nestle Health Science; A.L. reports a close family member employed by Merck & Co., Inc.; C.E.P. is an Associate Editor of Diabetes Care, receives payments from Wolters Kluwer for UpToDate chapters on diabetes in pregnancy and has received payments for consulting and speaking from Mediflix Inc; E.S. is a Deputy Editor of Diabetes Care and a member of the editorial board of Diabetologia and receives payments from Wolters Kluwer for chapters and laboratory monographs in UpToDate on measurements of glycemic control and screening tests for type 2 diabetes; W.H.H.S. has been an Advisor and/or Speaker for AstraZeneca, Bayer HealthCare, Boehringer Ingelheim Pharmaceuticals, Daiichi Sankyo, Eli Lilly and Company, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma Corporation, Novartis Pharmaceuticals, Novo Nordisk, Pfizer, Sanofi-Aventis, Takeda Pharmaceutical Company; J.M.W. reports a research collaboration with ENABLE Biosciences; R.J.B. has received research support from Amryt, Third Rock Ventures, Ionis and Regeneron; L.K.B. has received consulting honoraria from Bayer, Novo Nordisk, Sanofi, Lilly and Xeris; J.C.F. has received speaking honoraria from AstraZeneca and Novo Nordisk for scientific talks over which he had full control of content; A.L.G.’s spouse holds stock options in Roche and is an employee of Genentech; M.F.G. has received financial and nonfinancial (in kind) support from Boehringer Ingelheim Pharma GmbH, JDRF International, Eli Lilly, AbbVie, Sanofi-Aventis, Astellas, Novo Nordisk A/S, Bayer AG, within EU grant H2020-JTI-lMl2-2015-05 (grant agreement number 115974 - BEAt-DKD); and also from Novo Nordisk, Pfizer, Follicum, Coegin Pharma, Abcentra, Probi, Johnson & Johnson, within a project funded by the Swedish Foundation for Strategic Research on precision medicine in diabetes (LUDC-IRC no. 15-0067). M.F.G. has received personal consultancy fees from Lilly and Tribune Therapeutics AB. R.dS. has served as an external resource person to the World Health Organization’s Nutrition Guidelines Advisory Group on trans fats, saturated fats, and polyunsaturated fats. The WHO paid for his travel and accommodation to attend meetings from 2012 to 2017 to present and discuss this work. He has presented updates of this work to the WHO in 2022. He has also done contract research for the Canadian Institutes of Health Research’s Institute of Nutrition, Metabolism, and Diabetes, Health Canada, and the World Health Organization for which he received remuneration. He has received speaker’s fees from the University of Toronto, and McMaster Children’s Hospital. He has served as an independent director of the Helderleigh Foundation (Canada). He serves as a member of the Nutrition Science Advisory Committee to Health Canada (Government of Canada), co-chair of the Method working group of the ADA/EASD Precision Medicine in Diabetes group and is a co-opted member of the Scientific Advisory Committee on Nutrition (SACN) Subgroup on the Framework for the Evaluation of Evidence (Public Health England). He has held grants from the Canadian Institutes of Health Research, Canadian Foundation for Dietetic Research, Population Health Research Institute, and Hamilton Health Sciences Corporation as a principal investigator, and is a co-investigator on several funded team grants from the Canadian Institutes of Health Research. P.A.G. consults or has consulted for Provention Bio and Viacyte. He is the cofounder of IM Therapeutics and serves as CMO on the Board as well as being a shareholder. P.A.G. has also received research support from Helmsley Foundation, Nova Pharmaceuticals, Imcyse, Provention Bio, Intrexon T1D Partners and Novartis. All payments have been made to the University of Colorado; R.C.W.M. has received research grants from AstraZeneca, Bayer, Novo Nordisk, Pfizer, Roche Diagnostics (HK) Ltd, Tricida Inc, and consultancy/speaker honorarium from AstraZeneca, Boehringer Ingelheim, Bayer and Merck. All proceeds have been donated to the Chinese University of Hong Kong to support diabetes research. R.C.W.M. is a cofounder of GemVCare, a technology start-up initiated with support from the Hong Kong Government Innovation and Technology Commission and its Technology Start-up Support Scheme for Universities (TSSSU); C.M. serves or has served on the advisory panel for Novo Nordisk, Sanofi, Merck Sharp and Dohme Ltd., Eli Lilly and Company, Novartis, AstraZeneca, Boehringer Ingelheim, Roche, Medtronic, ActoBio Therapeutics, Pfizer, Imcyse, Insulet, Zealand Pharma, Avotres, Mannkind, Sandoz and Vertex. Financial compensation for these activities has been received by KU Leuven; KU Leuven has received research support for C.M. from Medtronic, Imcyse, Novo Nordisk, Sanofi and ActoBio Therapeutics; C.M. serves or has served on the speakers’ bureau for Novo Nordisk, Sanofi, Eli Lilly and Company, Boehringer Ingelheim, AstraZeneca and Novartis. Financial compensation for these activities has been received by KU Leuven. C.M. has been an Advisor and/or Speaker for Boehringer Ingelheim, Eli Lilly, Novo Nordisk and Sanofi-Aventis. C.M. is president of EASD (all external support of EASD is to be found on
Figures
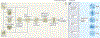
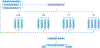
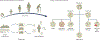
References
-
- Semnani-Azad Z et al. Predictors and risk factors of short-term and long-term outcomes among women with gestational diabetes mellitus (GDM) and their offspring: moving toward precision prognosis? Preprint at medRxiv 10.1101/2023.04.14.23288199 (2023). - DOI
Publication types
MeSH terms
Grants and funding
- R01 DK124806/DK/NIDDK NIH HHS/United States
- R21 DK125888/DK/NIDDK NIH HHS/United States
- T32 HL007024/HL/NHLBI NIH HHS/United States
- R03 DK127472/DK/NIDDK NIH HHS/United States
- IK2 CX001907/CX/CSRD VA/United States
- R01 DK104942/DK/NIDDK NIH HHS/United States
- K24 HL157960/HL/NHLBI NIH HHS/United States
- UM1 DK126185/DK/NIDDK NIH HHS/United States
- MC_UU_00014/4/MRC_/Medical Research Council/United Kingdom
- P30 DK040561/DK/NIDDK NIH HHS/United States
- U24 HD093486/HD/NICHD NIH HHS/United States
- MR/V034294/1/MRC_/Medical Research Council/United Kingdom
- P30 DK048520/DK/NIDDK NIH HHS/United States
- T32 DK007028/DK/NIDDK NIH HHS/United States
- K08 DK133676/DK/NIDDK NIH HHS/United States
- R01 HL130153/HL/NHLBI NIH HHS/United States
- K24 HL152440/HL/NHLBI NIH HHS/United States
- R01 DK125780/DK/NIDDK NIH HHS/United States
- R25 HL146166/HL/NHLBI NIH HHS/United States
- K23 DK133690/DK/NIDDK NIH HHS/United States
- K23 DK129821/DK/NIDDK NIH HHS/United States
- K23 DK114551/DK/NIDDK NIH HHS/United States
- R01 DK121843/DK/NIDDK NIH HHS/United States
- R00 HD108272/HD/NICHD NIH HHS/United States
- I01 BX001733/BX/BLRD VA/United States
- R01 DK124395/DK/NIDDK NIH HHS/United States
- U01 DK106993/DK/NIDDK NIH HHS/United States
- P30 DK072476/DK/NIDDK NIH HHS/United States
- UG1 HD107691/HD/NICHD NIH HHS/United States
- MR/W003740/1/MRC_/Medical Research Council/United Kingdom
- U01 DK127382/DK/NIDDK NIH HHS/United States
- K23 DK129799/DK/NIDDK NIH HHS/United States
- U01 HG007775/HG/NHGRI NIH HHS/United States
- P30 DK097512/DK/NIDDK NIH HHS/United States
- P30 DK036836/DK/NIDDK NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- R01 DK134955/DK/NIDDK NIH HHS/United States
- R01 DK117168/DK/NIDDK NIH HHS/United States
- U01 DK078616/DK/NIDDK NIH HHS/United States
- K99 HD108272/HD/NICHD NIH HHS/United States
- K12 DK133995/DK/NIDDK NIH HHS/United States
- R01 DK122586/DK/NIDDK NIH HHS/United States
- KL2 TR002490/TR/NCATS NIH HHS/United States
- RG/17/12/33167/BHF_/British Heart Foundation/United Kingdom
- R01 DK118403/DK/NIDDK NIH HHS/United States
- U54 DK118612/DK/NIDDK NIH HHS/United States
- K23 DK128572/DK/NIDDK NIH HHS/United States
- 201505/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- R01 HL151855/HL/NHLBI NIH HHS/United States
- UM1 DK078616/DK/NIDDK NIH HHS/United States
- IK2 CX002027/CX/CSRD VA/United States
- R01 DK034818/DK/NIDDK NIH HHS/United States
- K01 HL157658/HL/NHLBI NIH HHS/United States
- 210752/Z/18/Z/WT_/Wellcome Trust/United Kingdom

