Toxicity-specific peripheral blood T and B cell dynamics in anti-PD-1 and combined immune checkpoint inhibition
- PMID: 37794264
- PMCID: PMC10700442
- DOI: 10.1007/s00262-023-03541-0
Toxicity-specific peripheral blood T and B cell dynamics in anti-PD-1 and combined immune checkpoint inhibition
Abstract
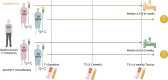
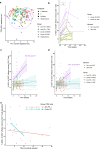
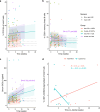
Immune checkpoint inhibitors (ICI) have revolutionized the treatment landscape of advanced malignancies, but come with a diverse spectrum of immune-related adverse events (irAEs). Mechanistic studies can aid the transition from expert-opinion to evidence-based irAE treatment strategies. We aimed to longitudinally characterize peripheral blood T and B cell dynamics in ICI-treated patients by multicolor flow cytometry and serum multiplex immunoassay at baseline, ± 3 weeks and ± 6 weeks or upon clinically relevant irAEs. We analyzed samples from 44 ICI-treated patients (24 anti-PD-1 monotherapy, 20 combined anti-PD-1/anti-CTLA-4; cICI), of whom 21 developed irAEs, and 10 healthy donors. IrAEs after cICI were characterized by significantly enhanced proliferation of Th1-associated, mainly (CD4+) CD27- effector memory T cells, as well as Th17-associated immune responses and germinal center activation (reflected by CXCL13 and IL-21 increases). We observed no changes in CD21lo, memory, class-switched or newly activated B cell subsets. Particularly double-positive PD-1+LAG-3+ CD8+ T cells showed enhanced cytotoxic capacity in patients with irAEs after cICI. Within anti-PD-1 monotherapy, irAEs were associated with modestly enhanced Th1-associated responses reflected by increased serum CXCL9 and CXCL10. In conclusion, ICI-induced toxicity is dominated by enhanced Th1-associated responses, but in cICI we also found evidence for Th17-associated responses and germinal center activation. Together, our data add to the growing body of evidence that irAEs may be driven by newly activated CD4+ helper T cells, specifically after cICI. This study also supports tailored irAE treatment, based on ICI regimen, and to deploy specific strategies such as Th17 inhibition especially in cICI-associated irAEs.
Keywords: Immune checkpoint inhibitors; Immune-related adverse event; Peripheral blood, immunophenotyping; T and B lymphocytes.
© 2023. The Author(s).
Conflict of interest statement
KPMS has advisory relationships with Bristol Myers Squibb, Novartis, MSD, Pierre Fabre, AbbVie, received honoraria from Novartis, MSD and Roche and received research funding from BMS, Philips and TigaTx. FW has advisory relationships with Janssen and Takeda, and received research funding from Takeda, Galapagos, BMS, Sanofi, and Leo Pharma.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

