Community-based management of a five-arm randomised clinical trial in COVID-19 outpatients in South Africa: challenges and opportunities
- PMID: 37794489
- PMCID: PMC10548657
- DOI: 10.1186/s13063-023-07577-6
Community-based management of a five-arm randomised clinical trial in COVID-19 outpatients in South Africa: challenges and opportunities
Abstract
Background: Repeated COVID-19 waves and corresponding mitigation measures have impacted health systems globally with exceptional challenges. In response to the pandemic, researchers, regulators, and funders rapidly pivoted to COVID-19 research activities. However, many clinical drug studies were not completed, due to often complex and rapidly evolving research conditions.
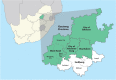
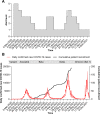
Methods: We outline our experience of planning and managing a randomised, adaptive, open-label, phase 2 clinical trial to evaluate the safety and efficacy of four repurposed drug regimens versus standard-of-care (SOC) in outpatients with 'mild to moderate' COVID-19 in Johannesburg, South Africa, in the context of a partnership with multiple stakeholders. The study was conducted between 3 September 2020 and 23 August 2021 during changing COVID-19 restrictions, significant morbidity and mortality waves, and allied supply line, economic, and political instability.
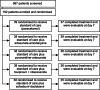
Results: Our clinical study design was pragmatic, including low-risk patients who were treated open label. There was built-in flexibility, including provision for some sample size adjustment and a range of secondary efficacy outcomes. Barriers to recruitment included the timing of waves, staff shortages due to illness, late presentation of patients, COVID-19 misinformation, and political unrest. Mitigations were the use of community health workers, deployment of mobile clinical units, and simplification of screening. Trial management required a radical reorganisation of logistics and processes to accommodate COVID-19 restrictions. These included the delivery of staff training and monitoring remotely, electronic consent, patient training and support to collect samples and report data at home, and the introduction of tele-medicine. These measures were successful for data collection, safe, and well received by patients.
Conclusion: Completing a COVID-19 trial in outpatients during the height of the pandemic required multiple innovations in nearly every aspect of clinical trial management, a high commitment level from study staff and patients, and support from study sponsors. Our experience has generated a more robust clinical research infrastructure, building in efficiencies to clinical trial management beyond the pandemic.
Keywords: COVID-19; Clinical research management; Clinical trial design; Drug repurposing; Outpatient; South Africa.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
NC declares grants and non-financial support from Shin Poong Pharm. Co. Ltd. during the conduct of the study and grants and non-financial support from ViiV Healthcare, Gilead Sciences, Merck, and Johnson & Johnson grants, personal fees and non-financial support from, personal fees from Cipla and Frontiers Biotech and Novo Nordisk outside the submitted work. NR reported personal fees from Medicines for Malaria Venture for the submitted work, and from Medicines for Malaria Venture, the World Health Organization, GlaxoSmithKline, and Artemida Pharma outside of the submitted work. ACM is an employee of Medicines for Malaria Venture. ACM holds share in Novartis, Alcon, and Idorsia. HJ was a consultant medical monitor for this study contracted by Medicines for Malaria Venture. JF was a consultant clinical trial manager for this study contracted by Medicines for Malaria Venture. WK and CJ are employees of Shin Poong Pharm. Co. Ltd. CJ has a patent pharmaceutical composition for COVID-19 treatment pending. SA-B is an employee of Artemida Pharma which received funding from Shin Poong Pharm. Co. Ltd. WDFV reports grants from Unitaid during the conduct of the study; grants from the Bill and Melinda Gates Foundation, the South African Medical Research Council, USAID, National Institutes of Health, FIND, Children’s Investment Fund Foundation; personal fees from Virology Education; grants, personal fees, and non-financial support from ViiV Healthcare; personal fees and non-financial support from Gilead Healthcare; grants, personal fees, and non-financial support from MSD; grants, personal fees, and non-financial support from Johnson & Johnson; and personal fees from Mylan/Viatris, Adcock-Ingram, Aspen, Abbott, Roche, and Sanofi outside the submitted work. CK, SN, NMa, YD, and NMu declare no competing interests.
Figures
Similar articles
-
Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study.Trials. 2021 Apr 2;22(1):245. doi: 10.1186/s13063-021-05157-0. Trials. 2021. PMID: 33810796 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Safety and efficacy of four drug regimens versus standard-of-care for the treatment of symptomatic outpatients with COVID-19: A randomised, open-label, multi-arm, phase 2 clinical trial.EBioMedicine. 2022 Dec;86:104322. doi: 10.1016/j.ebiom.2022.104322. Epub 2022 Nov 1. EBioMedicine. 2022. PMID: 36332361 Free PMC article. Clinical Trial.
-
Norwegian Coronavirus Disease 2019 (NO COVID-19) Pragmatic Open label Study to assess early use of hydroxychloroquine sulphate in moderately severe hospitalised patients with coronavirus disease 2019: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Jun 5;21(1):485. doi: 10.1186/s13063-020-04420-0. Trials. 2020. PMID: 32503662 Free PMC article.
-
Ivermectin for preventing and treating COVID-19.Cochrane Database Syst Rev. 2021 Jul 28;7(7):CD015017. doi: 10.1002/14651858.CD015017.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Jun 21;6:CD015017. doi: 10.1002/14651858.CD015017.pub3. PMID: 34318930 Free PMC article. Updated.
References
-
- Ghebreyesus T. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. 2020. Cited 2022 29 September. Available from: https://www.who.int/director-general/speeches/detail/who-director-genera....
-
- World Health Organization. Global excess deaths associated with COVID-19, January 2020 - December 2021. 2022. Cited 2022 13 October. Available from: https://www.who.int/data/stories/global-excess-deaths-associated-with-co....
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

