Clinical presentation of patients with lower limb spasticity undergoing routine treatment with botulinum toxin: baseline findings from an international observational study
- PMID: 37794845
- PMCID: PMC10562995
- DOI: 10.2340/jrm.v55.4257
Clinical presentation of patients with lower limb spasticity undergoing routine treatment with botulinum toxin: baseline findings from an international observational study
Abstract
Objective: Describe how people with lower limb spasticity present for treatment in routine clinical practice.
Methods: Prospective, observational study (Clinicaltrials.gov: NCT04050527) of ambulatory adult patients (≥ 18 years) with unilateral lower limb spasticity (able to take ≥ 5 steps with or without assistance) presenting for routine spasticity management, including treatment with abobotulinumtoxinA.
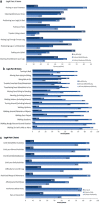
Results: The study population included 430 adults with lower limb spasticity. Despite their relatively young age (mean ± standard deviation 53.7 ± 13.9 years), only 20% of patients were employed. Most patients had an acquired brain injury due to cerebrovascular disease; 84.1% reported having concomitant upper limb spasticity. Using the Leg Activity Measure, most patients reported no or only mild difficulties in performing hygiene/positioning tasks, while 80.7% had at least mild difficulty with indoor ambulation and 90.5% had at least mild difficulty with walking outdoors. Sensory, communication and/or cognitive impairments were also common. At the first treatment cycle, 50.7% of patients set active function primary goals, including locomotion transferring or standing.
Conclusion: These observations highlight the complexity of presentation that must be considered when setting treatment goals for lower limb spasticity and emphasize the types of impairment and activity (functional) limitations that treating teams may expect to encounter in their patients and should cover in their initial and follow-up assessments.
Conflict of interest statement
AE, RZ, SA and JJ all received honoraria from Ipsen for undertaking this research. AE reports research funding from Ipsen, Allergan/AbbVie, and Merz, and consultancy for Ipsen, Allergan/AbbVie and Shionogi. SA has a specific interest in outcomes evaluation and has published extensively on the use of GAS in this context, as well as a number of the other standardized measures. All of these tools are freely available, however, and he has no personal financial interest in any of the material mentioned in this article. SA has received honoraria for lecturing, scientific advisory, peer training from Ipsen, Allergan and Merz and research funding from Ipsen. FCG, PM, and SP are employed by Ipsen. JJ has received honoraria for lecturing, scientific advisory, peer training from Ipsen, Allergan, and Merz.
Figures
References
-
- Javed M, Ali MH. Epidemiological burden of lower limb spasticity in adults: a systematic review. J Med Res Innov 2020; 4: e000195.
-
- Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, Rooney E, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc 2003; 51: 314–322. - PubMed
-
- Esquenazi, A. Assessment of spasticity and other consequences of the upper motor neuron syndrome affecting the lower limb. In: Spasticity, diagnosis and management. 2nd edition. A. Brashear (editor). New York, NY: Demos Medical Publishing; 2016, Chapter 8, 91–100.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical