Revisiting anti-Hu paraneoplastic autoimmunity: phenotypic characterization and cancer diagnosis
- PMID: 37794924
- PMCID: PMC10546956
- DOI: 10.1093/braincomms/fcad247
Revisiting anti-Hu paraneoplastic autoimmunity: phenotypic characterization and cancer diagnosis
Erratum in
-
Correction to: Revisiting anti-Hu paraneoplastic autoimmunity: phenotypic characterization and cancer diagnosis.Brain Commun. 2024 Dec 11;6(6):fcae387. doi: 10.1093/braincomms/fcae387. eCollection 2024. Brain Commun. 2024. PMID: 39664779 Free PMC article.
Abstract
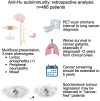
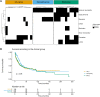
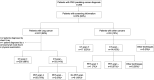
Anti-Hu are the most frequent antibodies in paraneoplastic neurological syndromes, mainly associated with an often limited stage small cell lung cancer. The clinical presentation is pleomorphic, frequently multifocal. Although the predominant phenotypes are well characterized, how different neurological syndromes associate is unclear. Likewise, no specific study assessed the performance of new-generation CT and PET scanners for cancer screening in these patients. Herein, we aimed to describe the clinical pattern and cancer screening in a retrospective cohort of 466 patients with anti-Hu autoimmunity from the French Reference Centre on Paraneoplastic Neurological Syndromes registry. Clinical presentation, cancer screening and diagnosis were analysed. Among the 466 patients, 220 (54%) had multifocal neurological involvement. A hierarchical cluster analysis grouped the patients into (i) mainly limbic encephalitis, (ii) predominantly peripheral neuropathy and (iii) broad involvement of the nervous system (mixed group). Compared with limbic encephalitis and mixed groups, patients in the neuropathy group more frequently had a chronic onset of symptoms (29 versus 13 and 17%), elevated CSF proteins (83 versus 47 and 67%) and died from cancer progression (67 versus 15 and 28%; all P < 0.05). No significant difference in overall survival was observed between groups. Dysautonomia and brainstem signs were associated with a higher risk of death from the neurological cause; cancer diagnosis was the main predictor of all-cause death, especially when diagnosed within 2 years from clinical onset (all P < 0.05). Three hundred and forty-nine (75%) patients had cancer: in 295 (84%) neurological symptoms preceded tumour diagnosis, being lung cancer in 262 (89%), thereof small cell lung cancer in 227 (87%). First CT scan revealed lung cancer in 205/241 (85%), and PET scan shortened the interval to diagnosis when the initial CT scan was negative [7 months (1-66) in 27 patients versus 14 months (2-45) in 6; P < 0.001]. Although cancer diagnosis mostly occurred within 2 years from clinical onset, 13/295 (4%) patients exceeded that threshold. Conversely, 33 patients (7%) were 'cancer-free' after 2 years of follow-up. However, 13/33 (39%) had initial suspicious imaging findings that spontaneously regressed. In conclusion, although anti-Hu autoimmunity clinical presentation is mostly multifocal, we observed patients with a predominant limbic syndrome or isolated sensory neuropathy. Early implementation of PET scan shortens the interval to cancer diagnosis, which was the strongest predictor of death, especially if diagnosed ≤2 years from clinical onset. As cancer was diagnosed >2 years after clinical onset in few patients, screening should be extended up to 5 years. In addition, tumour regression was suspected in a substantial proportion of 'cancer-free' patients.
Keywords: ANNA-1; anti-tumour immune response; cancer regression; clinical outcome; paraneoplastic autoimmunity.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures



References
-
- Giometto B, Grisold W, Vitaliani R, et al. . Paraneoplastic neurologic syndrome in the PNS Euronetwork database: A European study from 20 centers. Arch Neurol. 2010;67(3):330–335. - PubMed
-
- Graus F, Keime-Guibert F, Reñe R, et al. . Anti-Hu-associated paraneoplastic encephalomyelitis: Analysis of 200 patients. Brain. 2001;124(Pt 6):1138–1148. - PubMed
-
- Graus F, Cordon-Cardo C, Posner JB. Neuronal antinuclear antibody in sensory neuronopathy from lung cancer. Neurology. 1985;35(4):538–543. - PubMed
-
- Lucchinetti CF, Kimmel DW, Lennon VA. Paraneoplastic and oncologic profiles of patients seropositive for type 1 antineuronal nuclear autoantibodies. Neurology. 1998;50(3):652–657. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
