Short-Term Biomarker Modulation Study of Dasatinib for Estrogen Receptor-Negative Breast Cancer Chemoprevention
- PMID: 37795002
- PMCID: PMC10546803
- DOI: 10.4274/ejbh.galenos.2023.2023-7-3
Short-Term Biomarker Modulation Study of Dasatinib for Estrogen Receptor-Negative Breast Cancer Chemoprevention
Abstract
Objective: Risk-reducing therapy with selective estrogen receptor (ER) modulators and aromatase inhibitors reduce breast cancer risk. However, the effects are limited to ER-positive breast cancer. Therefore, new agents with improved toxicity profiles that reduce the risk in ER-negative breast cancers are urgently needed. The aim of this prospective, short-term, prevention study was to evaluate the effect of dasatinib, an inhibitor of the tyrosine kinase Src, on biomarkers in normal (but increased risk) breast tissue and serum of women at high risk for a second, contralateral primary breast cancer.
Materials and methods: Women with a history of unilateral stage I, II, or III ER-negative breast cancer, having no active disease, and who completed all adjuvant therapies were eligible. Patients underwent baseline fine-needle aspiration (FNA) of the contralateral breast and serum collection for biomarker analysis and were randomized to receive either no treatment (control) or dasatinib at 40 or 80 mg/day for three months. After three months, serum collection and breast FNA were repeated. Planned biomarker analysis consisted of changes in cytology and Ki-67 on breast FNA, and changes in serum levels of insulin-like growth factor 1 (IGF-1), IGF-binding protein 1, and IGF-binding protein 3. The primary objective was to evaluate changes in Ki-67 and secondary objective included changes in cytology in breast tissue and IGF-related serum biomarkers. Toxicity was also evaluated.
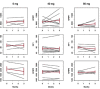
Results: Twenty-three patients started their assigned treatments. Compliance during the study was high, with 86.9% (20/23) of patients completing their assigned doses. Dasatinib was well tolerated and no drug-related grade 3 and 4 adverse events were observed. Since only one patient met the adequacy criteria for the paired FNA sample, we could not evaluate Ki-67 level or cytological changes. No significant change in serum biomarkers was observed among the three groups.
Conclusion: Dasatinib was well tolerated but did not induce any significant changes in serum biomarkers. The study could not fulfill its primary objective due to an inadequate number of paired FNA samples. Further, larger studies are needed to evaluate the effectiveness of Src inhibitors in breast cancer prevention.
Keywords: Chemoprevention; Src inhibitors; breast cancer risk.
©Copyright 2023 by the the Turkish Federation of Breast Diseases Societies / European Journal of Breast Health published by Galenos Publishing House.
Conflict of interest statement
Conflict of Interest: BA has received grant or research support from Susan G. Komen Breast Cancer Foundation. DY has received grant or research support from Susan G. Komen Breast Cancer Foundation and NIH/NCI. JKL received grant or research support from Medivation/Pfizer, Genentech, GSK, EMD-Sorono, Astra Zeneca, Zenith, Merck; participated in Speaker’s Bureau for MedLearning, Physician’s Education Resource, Clinical Care Options, WebMD, Tumor Board Tuesday, received Honoria from UpToDate; served on advisory committees or review panels for NCCN, ASCO, SITC Breast Committee. All other authors declare no relevant conflicts of interest.
Figures
References
-
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. - PubMed
-
- Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–2391. - PubMed
-
- Reichert D, Illiger HJ. [Primary prevention of breast cancer in women with an increased risk for breast cancer--a prospective, randomized, double-blind study (NSABP-P1 study)] Strahlenther Onkol. 2000;176:43–44. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous