Iatrogenic air embolism: pathoanatomy, thromboinflammation, endotheliopathy, and therapies
- PMID: 37795086
- PMCID: PMC10546929
- DOI: 10.3389/fimmu.2023.1230049
Iatrogenic air embolism: pathoanatomy, thromboinflammation, endotheliopathy, and therapies
Erratum in
-
Corrigendum: Iatrogenic air embolism: pathoanatomy, thromboinflammation, endotheliopathy, and therapies.Front Immunol. 2024 Feb 6;15:1378003. doi: 10.3389/fimmu.2024.1378003. eCollection 2024. Front Immunol. 2024. PMID: 38380313 Free PMC article.
Abstract
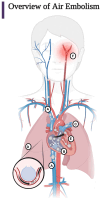
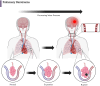
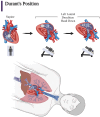
Iatrogenic vascular air embolism is a relatively infrequent event but is associated with significant morbidity and mortality. These emboli can arise in many clinical settings such as neurosurgery, cardiac surgery, and liver transplantation, but more recently, endoscopy, hemodialysis, thoracentesis, tissue biopsy, angiography, and central and peripheral venous access and removal have overtaken surgery and trauma as significant causes of vascular air embolism. The true incidence may be greater since many of these air emboli are asymptomatic and frequently go undiagnosed or unreported. Due to the rarity of vascular air embolism and because of the many manifestations, diagnoses can be difficult and require immediate therapeutic intervention. An iatrogenic air embolism can result in both venous and arterial emboli whose anatomic locations dictate the clinical course. Most clinically significant iatrogenic air emboli are caused by arterial obstruction of small vessels because the pulmonary gas exchange filters the more frequent, smaller volume bubbles that gain access to the venous circulation. However, there is a subset of patients with venous air emboli caused by larger volumes of air who present with more protean manifestations. There have been significant gains in the understanding of the interactions of fluid dynamics, hemostasis, and inflammation caused by air emboli due to in vitro and in vivo studies on flow dynamics of bubbles in small vessels. Intensive research regarding the thromboinflammatory changes at the level of the endothelium has been described recently. The obstruction of vessels by air emboli causes immediate pathoanatomic and immunologic and thromboinflammatory responses at the level of the endothelium. In this review, we describe those immunologic and thromboinflammatory responses at the level of the endothelium as well as evaluate traditional and novel forms of therapy for this rare and often unrecognized clinical condition.
Keywords: air embolism; arterioles; decompression sickness; hyperbaric oxygenation; microbubbles; thromboinflammation.
Copyright © 2023 Marsh, Moore, Moore, Bunch, Aboukhaled, Condon, Al-Fadhl, Thomas, Larson, Bower, Miller, Pearson, Twilling, Reser, Kim, Troyer, Yeager, Thomas, Srikureja, Patel, Añón, Thomas, Miller, Van Ryn, Pamulapati, Zimmerman, Wells, Martin, Seder, Aversa, Greene, March, Kwaan, Fulkerson, Vande Lune, Mollnes, Nielsen, Storm and Walsh.
Conflict of interest statement
EM and HM have received research grants from Haemonetics Corporation outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Storm BS. Venous Air Embolism and Complement-driven Thrombo inflammation. In vitro human whole blood studies and in vivo porcine studies on the effect of air emboli on the complement system, cytokine network, and the hemostasis. (2022). Available at: https://hdl.handle.net/10037/25922.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

