Optimization of universal allogeneic CAR-T cells combining CRISPR and transposon-based technologies for treatment of acute myeloid leukemia
- PMID: 37795087
- PMCID: PMC10546312
- DOI: 10.3389/fimmu.2023.1270843
Optimization of universal allogeneic CAR-T cells combining CRISPR and transposon-based technologies for treatment of acute myeloid leukemia
Abstract
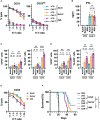
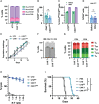
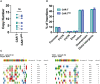
Despite the potential of CAR-T therapies for hematological malignancies, their efficacy in patients with relapse and refractory Acute Myeloid Leukemia has been limited. The aim of our study has been to develop and manufacture a CAR-T cell product that addresses some of the current limitations. We initially compared the phenotype of T cells from AML patients and healthy young and elderly controls. This analysis showed that T cells from AML patients displayed a predominantly effector phenotype, with increased expression of activation (CD69 and HLA-DR) and exhaustion markers (PD1 and LAG3), in contrast to the enriched memory phenotype observed in healthy donors. This differentiated and more exhausted phenotype was also observed, and corroborated by transcriptomic analyses, in CAR-T cells from AML patients engineered with an optimized CAR construct targeting CD33, resulting in a decreased in vivo antitumoral efficacy evaluated in xenograft AML models. To overcome some of these limitations we have combined CRISPR-based genome editing technologies with virus-free gene-transfer strategies using Sleeping Beauty transposons, to generate CAR-T cells depleted of HLA-I and TCR complexes (HLA-IKO/TCRKO CAR-T cells) for allogeneic approaches. Our optimized protocol allows one-step generation of edited CAR-T cells that show a similar phenotypic profile to non-edited CAR-T cells, with equivalent in vitro and in vivo antitumoral efficacy. Moreover, genomic analysis of edited CAR-T cells revealed a safe integration profile of the vector, with no preferences for specific genomic regions, with highly specific editing of the HLA-I and TCR, without significant off-target sites. Finally, the production of edited CAR-T cells at a larger scale allowed the generation and selection of enough HLA-IKO/TCRKO CAR-T cells that would be compatible with clinical applications. In summary, our results demonstrate that CAR-T cells from AML patients, although functional, present phenotypic and functional features that could compromise their antitumoral efficacy, compared to CAR-T cells from healthy donors. The combination of CRISPR technologies with transposon-based delivery strategies allows the generation of HLA-IKO/TCRKO CAR-T cells, compatible with allogeneic approaches, that would represent a promising option for AML treatment.
Keywords: AML; CRISPR; allogeneic CAR-T; transcriptomics (RNA sequencing); transposon.
Copyright © 2023 Calviño, Ceballos, Alfonso, Jauregui, Calleja-Cervantes, San Martin-Uriz, Rodriguez-Marquez, Martin-Mallo, Iglesias, Abizanda, Rodriguez-Diaz, Martinez-Turrillas, Illarramendi, Viguria, Redondo, Rifon, Villar, Lasarte, Inoges, Lopez-Diaz de Cerio, Hernaez, Prosper and Rodriguez-Madoz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

