Antihypertensive drug concentration measurement combined with personalized feedback in resistant hypertension: a randomized controlled trial
- PMID: 37796233
- PMCID: PMC10713002
- DOI: 10.1097/HJH.0000000000003585
Antihypertensive drug concentration measurement combined with personalized feedback in resistant hypertension: a randomized controlled trial
Abstract
Background: Adherence to antihypertensive drugs (AHDs) is crucial for controlling blood pressure (BP). We aimed to determine the effectiveness of measuring AHD concentrations using a dried blood spot (DBS) sampling method to identify nonadherence, combined with personalized feedback, in reducing resistant hypertension.
Methods: We conducted a multicenter, randomized, controlled trial (RHYME-RCT, ICTRP NTR6914) in patients with established resistant hypertension. Patients were randomized to receive either an intervention with standard of care (SoC) or SoC alone. SoC consisted of BP measurement and DBS sampling at baseline, 3 months (t3), 6 months (t6), and 12 months (t12); AHD concentrations were measured but not reported in this arm. In the intervention arm, results on AHD concentrations were discussed during a personalized feedback conversation at baseline and t3. Study endpoints included the proportion of patients with RH and AHD adherence at t12.
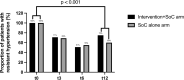
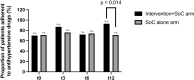
Results: Forty-nine patients were randomized to receive the intervention+SoC, and 51 were randomized to receive SoC alone. The proportion of adherent patients improved from 70.0 to 92.5% in the intervention+SoC arm ( P = 0.008, n = 40) and remained the same in the SoC arm (71.4%, n = 42). The difference in adherence between the arms was statistically significant ( P = 0.014). The prevalence of resistant hypertension decreased to 75.0% in the intervention+SoC arm ( P < 0.001, n = 40) and 59.5% in the SoC arm ( P < 0.001, n = 42) at t12; the difference between the arms was statistically nonsignificant ( P = 0.14).
Conclusion: Personalized feedback conversations based on DBS-derived AHD concentrations improved AHD adherence but did not reduce the prevalence of RH.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
L.E.J.P. has received lecture fees from Astellas Pharma. D.A.H. has received lecture fees and consulting fees from Astellas Pharma, Astra-Zeneca, Chiesi Pharma, Medincell, Novartis Pharma, Sangamo Therapeutics, and Vifor Pharma. He has received grant support from Astellas Pharma, Bristol-Myers Squibb, and Chiesi Pharma (paid to his institution). D.A.H. does not have employment or stock ownership at any of these companies, and neither does he have patents or patent applications. In the last 3 years, T.vG. has received lecture fees and study grants from Chiesi and Astellas, in addition to consulting fees from Roche Diagnostics, Thermo Fisher, Vitaeris, CSL Behring, Astellas, and Aurinia Pharma. In the last 3 years, L.vD. has received a research grant from Teva for a study not related to this one. The other authors declare no conflicts of interest.
Figures



Comment in
-
Consideration in the evaluation of follow-up of resistant hypertension.J Hypertens. 2024 Jan 1;42(1):56-57. doi: 10.1097/HJH.0000000000003603. Epub 2023 Nov 30. J Hypertens. 2024. PMID: 38033254 No abstract available.
-
Optimizing treatment outcomes: integrating antihypertensive drug concentration measurement, personalized feedback, and psychosocial factors in resistant hypertension.J Hypertens. 2024 Jun 1;42(6):1105-1106. doi: 10.1097/HJH.0000000000003691. Epub 2024 May 1. J Hypertens. 2024. PMID: 38690909 No abstract available.
References
-
- Hyman DJ, Pavlik V. Medication adherence and resistant hypertension. J Hum Hypertens 2015; 29:213–218. - PubMed
-
- Zeller A, Taegtmeyer A, Martina B, Battegay E, Tschudi P. Physicians’ ability to predict patients’ adherence to antihypertensive medication in primary care. Hypertens Res 2008; 31:1765–1771. - PubMed
-
- Burnier M. Drug adherence in hypertension. Pharmacol Res 2017; 124:1124–1140. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

