Leveraging the Expertise of the CTSA Program to Increase the Impact and Efficiency of Clinical Trials
- PMID: 37796498
- PMCID: PMC10773966
- DOI: 10.1001/jamanetworkopen.2023.36470
Leveraging the Expertise of the CTSA Program to Increase the Impact and Efficiency of Clinical Trials
Erratum in
-
Error in Byline.JAMA Netw Open. 2023 Nov 1;6(11):e2345388. doi: 10.1001/jamanetworkopen.2023.45388. JAMA Netw Open. 2023. PMID: 37948089 Free PMC article. No abstract available.
Abstract
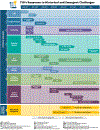
Importance: Multicenter clinical trials play a critical role in the translational processes that enable new treatments to reach all people and improve public health. However, conducting multicenter randomized clinical trials (mRCT) presents challenges. The Trial Innovation Network (TIN), established in 2016 to partner with the Clinical and Translational Science Award (CTSA) Consortium of academic medical institutions in the implementation of mRCTs, consists of 3 Trial Innovation Centers (TICs) and 1 Recruitment Innovation Center (RIC). This unique partnership has aimed to address critical roadblocks that impede the design and conduct of mRCTs, in expectation of accelerating the translation of novel interventions to clinical practice. The TIN's challenges and achievements are described in this article, along with examples of innovative resources and processes that may serve as useful models for other clinical trial networks providing operational and recruitment support.
Observations: The TIN has successfully integrated more than 60 CTSA institution program hubs into a functional network for mRCT implementation and optimization. A unique support system for investigators has been created that includes the development and deployment of novel tools, operational and recruitment services, consultation models, and rapid communication pathways designed to reduce delays in trial start-up, enhance recruitment, improve engagement of diverse research participants and communities, and streamline processes that improve the quality, efficiency, and conduct of mRCTs. These resources and processes span the clinical trial spectrum and enable the TICs and RIC to serve as coordinating centers, data centers, and recruitment specialists to assist trials across the National Institutes of Health and other agencies. The TIN's impact has been demonstrated through its response to both historical operational challenges and emerging public health emergencies, including the national opioid public health crisis and the COVID-19 pandemic.
Conclusions and relevance: The TIN has worked to reduce barriers to implementing mRCTs and to improve mRCT processes and operations by providing needed clinical trial infrastructure and resources to CTSA investigators. These resources have been instrumental in more quickly and efficiently translating research discoveries into beneficial patient treatments.
Conflict of interest statement
Drs Harris, Benjamin, Bernard, Dean, Dwyer, Ford, Selker, Wilkins, Huvane, Palm, Kimberly, Reilly, and Hanley; Mss Cook, Burr, Edwards, Kennedy, Lane, Nelson, Stroud, Thompson, and Busacca; and Mr Majkowski received support from grants from the National Center for Advancing Translational Sciences. Drs Ford, Selker, Palm, and Hanley; Ms Lane; and Mr Majkowski received support from grants from the National Institute on Aging. Dr Benjamin reported personal fees from Allergan, Melinta Therapeutics, and Syneos Health outside the submitted work. Dr Bernard reported grants from the National Institutes of Health Trial Innovation Center during the conduct of the study. Dr Dean reported grants from the National Institutes of Health during the conduct of the study. Dr Dwyer reported grants from Cincor and AstraZeneca; personal fees from Acuta, Akebia, Applied Therapeutics, Aurinia, Ardelyx, AstraZeneca, Bayer, Boehringer Ingelheim, Lilly, BioRasi, BioVie, Botanix, Caladrius, Cincor, ES Therapeutics, Fibrogen, Genentech, GSK, Inversago, Icon, Ionis, Keros, Medpace, MicRx, Novo Nordisk, ProKidney, PSI CRO, Rarestone, Reata, RenalytixAI, Sanofi, Tricida, ValenzaBio, and Worldwide Clinical Trials outside the submitted work; holds stock or options in Venostent, PathEx, Acelyrin, BioRasi, and Innovative Renal Care; and serves as President of the Collaborative Study Group and a Trustee of The Bolles School. Dr Wilkins reported grants from National Institutes of Health during the conduct of the study. Dr Huvane reported grants from the National Institutes of Health during the conduct of the study. Dr Elkind reported nonfinancial support from BMS-Pfizer and grants from Roche outside the submitted work. Dr Reilly reported personal fees from Novartis outside the submitted work. No other disclosures were reported.
Figures
Comment in
-
Clinical Trial Strategies Fueled by Informatics Innovation Catalyze Translational Research.JAMA Netw Open. 2023 Oct 2;6(10):e2336480. doi: 10.1001/jamanetworkopen.2023.36480. JAMA Netw Open. 2023. PMID: 37796508 No abstract available.
References
-
- Akobeng AK. Understanding randomised controlled trials. Arch Dis Child 2005;90:840–844. https://adc.bmj.com/content/90/8/840 - PMC - PubMed
-
- Marquis-Gravel G, Robertson H, Jones WS, et al. Streamlining the institutional review board process in pragmatic randomized clinical trials: challenges and lessons learned from the Aspirin Dosing: A Patient-centric Trial Assessing Benefits and Long-Term Effectiveness (ADAPTABLE) trial. Trials. 2021;22(1):90. doi: 10.1186/s13063-021-05026-w. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous