Airway surveillance and lung viral control by memory T cells induced by COVID-19 mRNA vaccine
- PMID: 37796612
- PMCID: PMC10721330
- DOI: 10.1172/jci.insight.172510
Airway surveillance and lung viral control by memory T cells induced by COVID-19 mRNA vaccine
Abstract
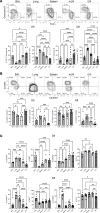
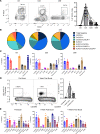
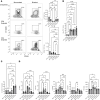
Although SARS-CoV-2 evolution seeds a continuous stream of antibody-evasive viral variants, COVID-19 mRNA vaccines provide robust protection against severe disease and hospitalization. Here, we asked whether mRNA vaccine-induced memory T cells limit lung SARS-CoV-2 replication and severe disease. We show that mice and humans receiving booster BioNTech mRNA vaccine developed potent CD8 T cell responses and showed similar kinetics of expansion and contraction of granzyme B/perforin-expressing effector CD8 T cells. Both monovalent and bivalent mRNA vaccines elicited strong expansion of a heterogeneous pool of terminal effectors and memory precursor effector CD8 T cells in spleen, inguinal and mediastinal lymph nodes, pulmonary vasculature, and most surprisingly in the airways, suggestive of systemic and regional surveillance. Furthermore, we document that: (a) CD8 T cell memory persists in multiple tissues for > 200 days; (b) following challenge with pathogenic SARS-CoV-2, circulating memory CD8 T cells rapidly extravasate to the lungs and promote expeditious viral clearance, by mechanisms that require CD4 T cell help; and (c) adoptively transferred splenic memory CD8 T cells traffic to the airways and promote lung SARS-CoV-2 clearance. These findings provide insights into the critical role of memory T cells in preventing severe lung disease following breakthrough infections with antibody-evasive SARS-CoV-2 variants.
Keywords: COVID-19; Cellular immune response; Immunology; Memory; T cells.
Figures




References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

