Multigene panel next generation sequencing in metastatic colorectal cancer in an Australian population
- PMID: 37796807
- PMCID: PMC10553362
- DOI: 10.1371/journal.pone.0292087
Multigene panel next generation sequencing in metastatic colorectal cancer in an Australian population
Abstract
Background: Next generation sequencing (NGS) is increasingly used in standard clinical practice to identify patients with potentially actionable mutations. Stratification of NGS mutation tiers is currently based on the European Society of Medical Oncology (ESMO) Scale for Clinical Actionability of Molecular Targets (ESCAT[E]) Tier I-V & X. Allele frequency is also increasingly recognised as an important prognostic tool in advanced cancer. The aim of this study was to determine the genomic mutations in metastatic colorectal cancer (CRC) in an Australian multicultural population and their influence on survival outcomes.
Methods: Next generation sequencing with the 50-gene panel Oncomine Precision Assay™ was used on 180 CRC tissue samples obtained across six Sydney hospitals between June 2021 and March 2022.
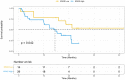
Results: From 180 samples, 147 (82%) had at least one gene mutation identified with 68 (38%) having two or more concurrent mutations. Tier I variants included RAS wild-type [EI] in 73 (41%) and BRAF V600E [EIA] in 27 (15%). Non-tier I variants include 2 (1%) ERBB2 amplification [EIIB], 26 (15%) PIK3CA hotspot mutations [EIIIA] and 9 (5%) MET focal amplifications [EIIIA]. NGS testing revealed an additional 22% of cases with Tier II & III mutations. 43% of patients also presented with potentially actionable Tier III & IV mutations. Patients with concurrent TP53 and RAS mutations had significantly reduced overall survival (6.1 months versus 21.1 months, p <0.01). High KRAS allele frequency, as defined by those with over 20% variant allele frequency (VAF), also demonstrated reduced overall survival (12.1 months versus 42.9 months, p = 0.04).
Conclusions: In addition to identifying patients with genomic alterations suitable for clinically proven standard of care therapeutic options, the 50 gene NGS panel has significant potential in identifying potentially actionable non-tier 1 mutations and therefore may become future standard clinical practice.
Copyright: © 2023 Nindra et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28(31):4697–705. doi: 10.1200/JCO.2009.27.4860 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

