A modified method to analyse cell proliferation using EdU labelling in large insect brains
- PMID: 37796816
- PMCID: PMC10553331
- DOI: 10.1371/journal.pone.0292009
A modified method to analyse cell proliferation using EdU labelling in large insect brains
Erratum in
-
Correction: A modified method to analyse cell proliferation using EdU labelling in large insect brains.PLoS One. 2024 Aug 29;19(8):e0309825. doi: 10.1371/journal.pone.0309825. eCollection 2024. PLoS One. 2024. PMID: 39208163 Free PMC article.
Abstract
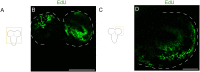
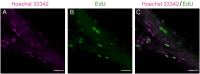
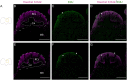
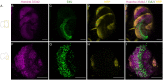
The study of neurogenesis is critical to understanding of the evolution of nervous systems. Within invertebrates, this process has been extensively studied in Drosophila melanogaster, which is the predominant model thanks to the availability of advanced genetic tools. However, insect nervous systems are extremely diverse, and by studying a range of taxa we can gain additional information about how nervous systems and their development evolve. One example of the high diversity of insect nervous system diversity is provided by the mushroom bodies. Mushroom bodies have critical roles in learning and memory and vary dramatically across species in relative size and the type(s) of sensory information they process. Heliconiini butterflies provide a useful snapshot of this diversity within a closely related clade. Within Heliconiini, the genus Heliconius contains species where mushroom bodies are 3-4 times larger than other closely related genera, relative to the rest of the brain. This variation in size is largely explained by increases in the number of Kenyon cells, the intrinsic neurons which form the mushroom body. Hence, variation in mushroom body size is the product of changes in cell proliferation during Kenyon cell neurogenesis. Studying this variation requires adapting labelling techniques for use in less commonly studied organisms, as methods developed for common laboratory insects often do not work. Here, we present a modified protocol for EdU staining to examine neurogenesis in large-brained insects, using Heliconiini butterflies as our primary case, but also demonstrating applicability to cockroaches, another large-brained insect.
Copyright: © 2023 Anton et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Campos-Ortega JA. Early Neurogenesis in Drosophila. Development. 1999; 331–345. doi: 10.1007/978-3-642-59828-9_20 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

