Protective effect of pre-existing natural immunity in a nonhuman primate reinfection model of congenital cytomegalovirus infection
- PMID: 37796819
- PMCID: PMC10553354
- DOI: 10.1371/journal.ppat.1011646
Protective effect of pre-existing natural immunity in a nonhuman primate reinfection model of congenital cytomegalovirus infection
Abstract
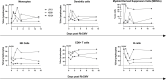
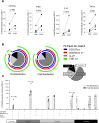
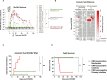
Congenital cytomegalovirus (cCMV) is the leading infectious cause of neurologic defects in newborns with particularly severe sequelae in the setting of primary CMV infection in the first trimester of pregnancy. The majority of cCMV cases worldwide occur after non-primary infection in CMV-seropositive women; yet the extent to which pre-existing natural CMV-specific immunity protects against CMV reinfection or reactivation during pregnancy remains ill-defined. We previously reported on a novel nonhuman primate model of cCMV in rhesus macaques where 100% placental transmission and 83% fetal loss were seen in CD4+ T lymphocyte-depleted rhesus CMV (RhCMV)-seronegative dams after primary RhCMV infection. To investigate the protective effect of preconception maternal immunity, we performed reinfection studies in CD4+ T lymphocyte-depleted RhCMV-seropositive dams inoculated in late first / early second trimester gestation with RhCMV strains 180.92 (n = 2), or RhCMV UCD52 and FL-RhCMVΔRh13.1/SIVgag, a wild-type-like RhCMV clone with SIVgag inserted as an immunological marker, administered separately (n = 3). An early transient increase in circulating monocytes followed by boosting of the pre-existing RhCMV-specific CD8+ T lymphocyte and antibody response was observed in the reinfected dams but not in control CD4+ T lymphocyte-depleted dams. Emergence of SIV Gag-specific CD8+ T lymphocyte responses in macaques inoculated with the FL-RhCMVΔRh13.1/SIVgag virus confirmed reinfection. Placental transmission was detected in only one of five reinfected dams and there were no adverse fetal sequelae. Viral whole genome, short-read, deep sequencing analysis confirmed transmission of both reinfection RhCMV strains across the placenta with ~30% corresponding to FL-RhCMVΔRh13.1/SIVgag and ~70% to RhCMV UCD52, consistent with the mixed human CMV infections reported in infants with cCMV. Our data showing reduced placental transmission and absence of fetal loss after non-primary as opposed to primary infection in CD4+ T lymphocyte-depleted dams indicates that preconception maternal CMV-specific CD8+ T lymphocyte and/or humoral immunity can protect against cCMV infection.
Copyright: © 2023 Moström et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
“I have read the journal’s policy and the authors of this manuscript have the following competing interests: Sallie Permar serves as a consultant to GSK, Moderna, Merck, Pfizer, Hoopika, and Dynavax vaccine programs, as well as leading a sponsored research program with Moderna and Merck. Oregon Health Sciences University (OHSU), Klaus Früh, Daniel Malouli and Scott Hansen have a substantial financial interest in Vir Biotechnology, Inc. a company that may have a commercial interest in the results of this research and technology. Klaus Früh, Daniel Malouli and Scott Hansen are co-inventors of several patents licensed to Vir Biotechnology. Klaus Früh and Scott Hansen are also consultants to Vir Biotechnology, Inc. All potential conflicts of interest have been reviewed and managed by OHSU. All other authors report no potential conflicts”
Figures




Update of
-
Protective effect of pre-existing natural immunity in a nonhuman primate reinfection model of congenital cytomegalovirus infection.bioRxiv [Preprint]. 2023 Apr 10:2023.04.10.536057. doi: 10.1101/2023.04.10.536057. bioRxiv. 2023. Update in: PLoS Pathog. 2023 Oct 5;19(10):e1011646. doi: 10.1371/journal.ppat.1011646. PMID: 37090643 Free PMC article. Updated. Preprint.
Similar articles
-
Protective effect of pre-existing natural immunity in a nonhuman primate reinfection model of congenital cytomegalovirus infection.bioRxiv [Preprint]. 2023 Apr 10:2023.04.10.536057. doi: 10.1101/2023.04.10.536057. bioRxiv. 2023. Update in: PLoS Pathog. 2023 Oct 5;19(10):e1011646. doi: 10.1371/journal.ppat.1011646. PMID: 37090643 Free PMC article. Updated. Preprint.
-
Relationship of maternal cytomegalovirus-specific antibody responses and viral load to vertical transmission risk following primary maternal infection in a rhesus macaque model.PLoS Pathog. 2023 Oct 23;19(10):e1011378. doi: 10.1371/journal.ppat.1011378. eCollection 2023 Oct. PLoS Pathog. 2023. PMID: 37871009 Free PMC article.
-
Relationship of maternal cytomegalovirus-specific antibody responses and viral load to vertical transmission risk following primary maternal infection in a rhesus macaque model.bioRxiv [Preprint]. 2023 Apr 21:2023.04.21.537769. doi: 10.1101/2023.04.21.537769. bioRxiv. 2023. Update in: PLoS Pathog. 2023 Oct 23;19(10):e1011378. doi: 10.1371/journal.ppat.1011378. PMID: 37131785 Free PMC article. Updated. Preprint.
-
The Current Challenges in Developing Biological and Clinical Predictors of Congenital Cytomegalovirus Infection.Int J Mol Sci. 2021 Dec 15;22(24):13487. doi: 10.3390/ijms222413487. Int J Mol Sci. 2021. PMID: 34948284 Free PMC article. Review.
-
Human Cytomegalovirus Infection in Women With Preexisting Immunity: Sources of Infection and Mechanisms of Infection in the Presence of Antiviral Immunity.J Infect Dis. 2020 Mar 5;221(Suppl 1):S1-S8. doi: 10.1093/infdis/jiz464. J Infect Dis. 2020. PMID: 32134479 Free PMC article. Review.
Cited by
-
Pathogenesis of viral infections during pregnancy.Clin Microbiol Rev. 2024 Jun 13;37(2):e0007323. doi: 10.1128/cmr.00073-23. Epub 2024 Feb 29. Clin Microbiol Rev. 2024. PMID: 38421182 Free PMC article. Review.
-
Preexisting vaccine-primed heterosubtypic T cell immunity protects the maternal-fetal unit from adverse influenza outcomes in mice.J Clin Invest. 2025 Jan 2;135(1):e179230. doi: 10.1172/JCI179230. J Clin Invest. 2025. PMID: 39744951 Free PMC article.
-
Innate immune responses to pathogens at the maternal-fetal interface.Nat Rev Immunol. 2025 Jun 18. doi: 10.1038/s41577-025-01191-0. Online ahead of print. Nat Rev Immunol. 2025. PMID: 40533582 Review.
-
Nonhuman primate models of pediatric viral diseases.Front Cell Infect Microbiol. 2024 Dec 3;14:1493885. doi: 10.3389/fcimb.2024.1493885. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39691699 Free PMC article. Review.
-
Replication-deficient whole-virus vaccines against cytomegalovirus induce protective immunity in a guinea pig congenital infection model.J Virol. 2025 Jul 22;99(7):e0020725. doi: 10.1128/jvi.00207-25. Epub 2025 Jun 11. J Virol. 2025. PMID: 40497723 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

