A next-generation intranasal trivalent MMS vaccine induces durable and broad protection against SARS-CoV-2 variants of concern
- PMID: 37796985
- PMCID: PMC10576135
- DOI: 10.1073/pnas.2220403120
A next-generation intranasal trivalent MMS vaccine induces durable and broad protection against SARS-CoV-2 variants of concern
Abstract
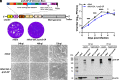
As SARS-CoV-2 variants of concern (VoCs) that evade immunity continue to emerge, next-generation adaptable COVID-19 vaccines which protect the respiratory tract and provide broader, more effective, and durable protection are urgently needed. Here, we have developed one such approach, a highly efficacious, intranasally delivered, trivalent measles-mumps-SARS-CoV-2 spike (S) protein (MMS) vaccine candidate that induces robust systemic and mucosal immunity with broad protection. This vaccine candidate is based on three components of the MMR vaccine, a measles virus Edmonston and the two mumps virus strains [Jeryl Lynn 1 (JL1) and JL2] that are known to provide safe, effective, and long-lasting protective immunity. The six proline-stabilized prefusion S protein (preS-6P) genes for ancestral SARS-CoV-2 WA1 and two important SARS-CoV-2 VoCs (Delta and Omicron BA.1) were each inserted into one of these three viruses which were then combined into a trivalent "MMS" candidate vaccine. Intranasal immunization of MMS in IFNAR1-/- mice induced a strong SARS-CoV-2-specific serum IgG response, cross-variant neutralizing antibodies, mucosal IgA, and systemic and tissue-resident T cells. Immunization of golden Syrian hamsters with MMS vaccine induced similarly high levels of antibodies that efficiently neutralized SARS-CoV-2 VoCs and provided broad and complete protection against challenge with any of these VoCs. This MMS vaccine is an efficacious, broadly protective next-generation COVID-19 vaccine candidate, which is readily adaptable to new variants, built on a platform with a 50-y safety record that also protects against measles and mumps.
Keywords: MMR vaccine; SARS-CoV-2; intranasal trivalent vaccine.
Conflict of interest statement
The Ohio State University Research Foundation has filed a patent application for the measles virus-based SARS-CoV-2 vaccine candidates. In addition, The Ohio State University Research Foundation and Abigail Wexner Research Institute at Nationwide Children’s Hospital have filed a patent application for the mumps virus–based SARS-CoV-2 vaccine candidates.
Figures










References
-
- Krammer F., SARS-CoV-2 vaccines in development. Nature 586, 516–527 (2020). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- U54 CA260582/CA/NCI NIH HHS/United States
- U19 AI142731/AI/NIAID NIH HHS/United States
- R01 AI090060/AI/NIAID NIH HHS/United States
- R01 HL168501/HL/NHLBI NIH HHS/United States
- P01 AI112524/AI/NIAID NIH HHS/United States
- R01 AI157205/AI/NIAID NIH HHS/United States
- R01 HL154001/HL/NHLBI NIH HHS/United States
- R01 AI153829/AI/NIAID NIH HHS/United States
- R56 AI166587/AI/NIAID NIH HHS/United States
- R01 AI145144/AI/NIAID NIH HHS/United States
- T32 AI165391/AI/NIAID NIH HHS/United States
- R01 AI130110/AI/NIAID NIH HHS/United States
- R01 HL157215/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

