Safety, Effectiveness, and Hemodynamic Performance of the Bovine Pericardium Organic Valvular Bioprosthesis
- PMID: 37797247
- PMCID: PMC10549993
- DOI: 10.21470/1678-9741-2023-0015
Safety, Effectiveness, and Hemodynamic Performance of the Bovine Pericardium Organic Valvular Bioprosthesis
Abstract
Objective: To assess actual data on the safety, effectiveness, and hemodynamic performance of Bovine Pericardium Organic Valvular Bioprosthesis (BVP).
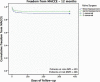
Methods: The BIOPRO Trial is an observational, retrospective, non-comparative, non-randomized, and multicenter study. We collected data from 903 patients with symptomatic, moderate, or severe valve disease who underwent BVP implants in the timeframe from 2013 to 2020 at three Brazilian institutions. Death, valve-related adverse events (AEs), functional recovery, and hemodynamic performance were evaluated at the hospital, at discharge, and six months and one year later. Primary analysis compared late (> 30 days after implant) linearized rates of valve-related AEs, such as thromboembolism, valve thrombosis, major hemorrhage, major paravalvular leak, and endocarditis, following objective performance criteria (OPC). Analysis was performed to include at least 400 valve-years for each valve position (aortic and mitral) for complete comparisons to OPC. Kaplan-Meier survival and major adverse cardiovascular and cerebrovascular event analyses were also performed.
Results: This retrospective study analyzed follow-up data collected from 903 patients (834.2 late patient-years) who have undergone surgery for 455 isolated aortic valve replacement (50.4%), 382 isolated mitral valve replacement (42.3%), and 66 combined valve replacement or other intervention (7.3%). The linearized rates of valve-related AEs were < 2 × OPC. One-year survival rates were 95.1% and 92.7% for aortic and mitral valve replacement, respectively. This study demonstrated an improvement in the New York Heart Association classification from baseline and hemodynamic performance within an expected range.
Conclusion: According to this analysis, BVP meets world standards for safety and clinical efficacy.
Keywords: Animals; Aortic Valve; Bioprosthesis; Heart Valve Prosthesis; Hemodynamics; Prosthesis Design; Treatment Outcomes.
Conflict of interest statement
No conflict of interest.
Figures








References
-
- Singhal P, Luk A, Butany J. Bioprosthetic heart valves: impact of implantation on biomaterials. ISRN Biomaterials. 2013:1–14. doi: 10.5402/2013/728791. - DOI
-
- Bapat VN. The pericardial heart valve: the odyssey of a continuously evolving concept (1971-2014) [Internet] Society for Cardiothoracic Surgery in Great Britain and Ireland; c2014. pp. 133–133. [cited 2023 Mar 12]. Available from: https://www.ctsnet.org/news/pericardial-heart-valve-book-now-available-c....
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
