Plasma neurofilament light chain predicts Alzheimer's disease in patients with subjective cognitive decline and mild cognitive impairment: A cross-sectional and longitudinal study
- PMID: 37797300
- PMCID: PMC11235835
- DOI: 10.1111/ene.16089
Plasma neurofilament light chain predicts Alzheimer's disease in patients with subjective cognitive decline and mild cognitive impairment: A cross-sectional and longitudinal study
Abstract
Background and purpose: We aimed to evaluate the accuracy of plasma neurofilament light chain (NfL) in predicting Alzheimer's disease (AD) and the progression of cognitive decline in patients with subjective cognitive decline (SCD) and mild cognitive impairment (MCI).
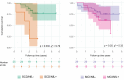
Methods: This longitudinal cohort study involved 140 patients (45 with SCD, 73 with MCI, and 22 with AD dementia [AD-D]) who underwent plasma NfL and AD biomarker assessments (cerebrospinal fluid, amyloid positron emission tomography [PET], and 18 F-fluorodeoxyglucose-PET) at baseline. The patients were rated according to the amyloid/tau/neurodegeneration (A/T/N) system and followed up for a mean time of 2.72 ± 0.95 years to detect progression from SCD to MCI and from MCI to AD. Forty-eight patients (19 SCD, 29 MCI) also underwent plasma NfL measurements 2 years after baseline.
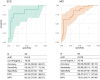
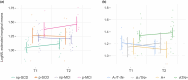
Results: At baseline, plasma NfL detected patients with biomarker profiles consistent with AD (A+/T+/N+ or A+/T+/N-) with high accuracy (area under the curve [AUC] 0.82). We identified cut-off values of 19.45 pg/mL for SCD and 20.45 pg/mL for MCI. During follow-up, nine SCD patients progressed to MCI (progressive SCD [p-SCD]), and 14 MCI patients developed AD dementia (progressive MCI [p-MCI]). The previously identified cut-off values provided good accuracy in identifying p-SCD (80% [95% confidence interval 65.69: 94.31]). The rate of NfL change was higher in p-MCI (3.52 ± 4.06 pg/mL) compared to non-progressive SCD (0.81 ± 1.25 pg/mL) and non-progressive MCI (-0.13 ± 3.24 pg/mL) patients. A rate of change lower than 1.64 pg/mL per year accurately excluded progression from MCI to AD (AUC 0.954).
Conclusion: Plasma NfL concentration and change over time may be a reliable, non-invasive tool to detect AD and the progression of cognitive decline at the earliest stages of the disease.
Keywords: Alzheimer's disease; biomarker; dementia; mild cognitive impairment; neurofilament.
© 2023 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
All the authors declared that they have no conflicts of interest relevant to this work.
Figures

References
-
- Margolis SA, Kelly DA, Daiello LA, et al. Anticholinergic/sedative drug burden and subjective cognitive decline in older adults at risk of Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 2021;76:1037‐1043. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

