Utilization of an Artery-on-a-Chip to Unravel Novel Regulators and Therapeutic Targets in Vascular Diseases
- PMID: 37797407
- PMCID: PMC11468405
- DOI: 10.1002/adhm.202302907
Utilization of an Artery-on-a-Chip to Unravel Novel Regulators and Therapeutic Targets in Vascular Diseases
Abstract
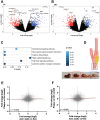
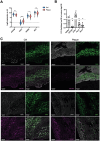
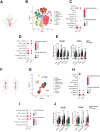
In this study, organ-on-chip technology is used to develop an in vitro model of medium-to-large size arteries, the artery-on-a-chip (AoC), with the objective to recapitulate the structure of the arterial wall and the relevant hemodynamic forces affecting luminal cells. AoCs exposed either to in vivo-like shear stress values or kept in static conditions are assessed to generate a panel of novel genes modulated by shear stress. Considering the crucial role played by shear stress alterations in carotid arteries affected by atherosclerosis (CAD) and abdominal aortic aneurysms (AAA) disease development/progression, a patient cohort of hemodynamically relevant specimens is utilized, consisting of diseased and non-diseased (internal control) vessel regions from the same patient. Genes activated by shear stress follow the same expression pattern in non-diseased segments of human vessels. Single cell RNA sequencing (scRNA-seq) enables to discriminate the unique cell subpopulations between non-diseased and diseased vessel portions, revealing an enrichment of flow activated genes in structural cells originating from non-diseased specimens. Furthermore, the AoC served as a platform for drug-testing. It reproduced the effects of a therapeutic agent (lenvatinib) previously used in preclinical AAA studies, therefore extending the understanding of its therapeutic effect through a multicellular structure.
Keywords: aortic aneurysms; arteries-on-a-chip; atherosclerosis; endothelial cells; smooth muscle cells; vascular diseases.
© 2023 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
We declare the following competing interest: Lars Maegdefessel is a scientific consultant and adviser for Novo Nordisk (Malov, Denmark), DrugFarm (Shanghai, China), and Angiolutions (Hannover, Germany), and received research funds from Roche Diagnostics (Rotkreuz, Switzerland).
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

