Loss of carnitine palmitoyltransferase 1a reduces docosahexaenoic acid-containing phospholipids and drives sexually dimorphic liver disease in mice
- PMID: 37797918
- PMCID: PMC10568566
- DOI: 10.1016/j.molmet.2023.101815
Loss of carnitine palmitoyltransferase 1a reduces docosahexaenoic acid-containing phospholipids and drives sexually dimorphic liver disease in mice
Abstract
Background and aims: Genome and epigenome wide association studies identified variants in carnitine palmitoyltransferase 1a (CPT1a) that associate with lipid traits. The goal of this study was to determine the role of liver-specific CPT1a on hepatic lipid metabolism.
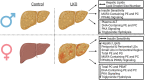
Approach and results: Male and female liver-specific knockout (LKO) and littermate controls were placed on a low-fat or high-fat diet (60% kcal fat) for 15 weeks. Mice were necropsied after a 16 h fast, and tissues were collected for lipidomics, matrix-assisted laser desorption ionization mass spectrometry imaging, kinome analysis, RNA-sequencing, and protein expression by immunoblotting. Female LKO mice had increased serum alanine aminotransferase levels which were associated with greater deposition of hepatic lipids, while male mice were not affected by CPT1a deletion relative to male control mice. Mice with CPT1a deletion had reductions in DHA-containing phospholipids at the expense of monounsaturated fatty acids (MUFA)-containing phospholipids in whole liver and at the level of the lipid droplet (LD). Male and female LKO mice increased RNA levels of genes involved in LD lipolysis (Plin2, Cidec, G0S2) and in polyunsaturated fatty acid metabolism (Elovl5, Fads1, Elovl2), while only female LKO mice increased genes involved in inflammation (Ly6d, Mmp12, Cxcl2). Kinase profiling showed decreased protein kinase A activity, which coincided with increased PLIN2, PLIN5, and G0S2 protein levels and decreased triglyceride hydrolysis in LKO mice.
Conclusions: Liver-specific deletion of CPT1a promotes sexually dimorphic steatotic liver disease (SLD) in mice, and here we have identified new mechanisms by which females are protected from HFD-induced liver injury.
Keywords: Fatty acid metabolism; Lipid droplets; Lipolysis; Mitochondria; Triglycerides.
Copyright © 2023 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Update of
-
Loss of Carnitine Palmitoyltransferase 1a Reduces Docosahexaenoic Acid-Containing Phospholipids and Drives Sexually Dimorphic Liver Disease in Mice.bioRxiv [Preprint]. 2023 Aug 18:2023.08.17.553705. doi: 10.1101/2023.08.17.553705. bioRxiv. 2023. Update in: Mol Metab. 2023 Dec;78:101815. doi: 10.1016/j.molmet.2023.101815. PMID: 37645721 Free PMC article. Updated. Preprint.
References
-
- Rinella M.E., Lazarus J.V., Ratziu V., Francque S.M., Sanyal A.J., Kanwal F., et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023 Online ahead of print. PMID: 37363821. - PubMed
-
- Burra P., Bizzaro D., Gonta A., Shalaby S., Gambato M., Morelli M.C., et al. Clinical impact of sexual dimorphism in non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) Liver Int. 2021;41:1713–1733. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

