Euglycemic diabetic ketoacidosis in the era of SGLT-2 inhibitors
- PMID: 37797963
- PMCID: PMC10551972
- DOI: 10.1136/bmjdrc-2023-003666
Euglycemic diabetic ketoacidosis in the era of SGLT-2 inhibitors
Abstract
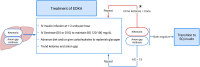
Euglycemic diabetic ketoacidosis (EDKA) is an emerging complication of diabetes associated with an increasing use of sodium-glucose transporter type 2 (SGLT-2) inhibitor drugs. This review highlights the growing incidence of EDKA and its diagnostic challenges due to the absence of hallmark hyperglycemia seen in diabetic ketoacidosis (DKA). The paper presents a classification system for the severity of EDKA, categorizing it into mild, moderate, and severe based on serum pH and bicarbonate levels. Another classification system is proposed to define stages of EDKA based on anion gap and ketones at the time of diagnosis and during the treatment period. A treatment algorithm is proposed to guide clinicians in managing EDKA. This treatment algorithm includes monitoring anion gap and ketones to guide insulin and fluid management, and slower transition to subcutaneous insulin to prevent a relapse. Increased awareness of EDKA is essential for a timely diagnosis because an early diagnosis and treatment can improve clinical outcomes.
Keywords: diabetic ketoacidosis; drug-related side effects and adverse reactions; ketoacidosis.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- Bilgin S, Kurtkulagi O, Duman TT, et al. Sodium glucose co-transporter-2 inhibitor, empagliflozin, is associated with significant reduction in weight, body mass index, fasting glucose, and A1C levels in type 2 diabetic patients with established coronary heart disease: the SUPER GATE study. Ir J Med Sci 2022;191:1647–52. 10.1007/s11845-021-02761-6 - DOI - PMC - PubMed
-
- European Medicines Agency . Review of diabetes medicines called Sglt2 inhibitors started: risk of diabetic ketoacidosis to be examined. 2015. Available: https://www.ema.europa.eu/en/documents/referral/sglt2-inhibitors-article... [Accessed 31 Jul 2023].
-
- Health Canada. Forxiga, Invokana . Health Canada begins safety review of diabetes drugs known as SGLT2 inhibitors and risk of ketoacidosis. Ottawa, ON: Health Canada, 2015. Available: https://www.canada.ca/en/health-canada/services/drugs-health-products/me...
-
- US Food Drug Administration . FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. Drug safety communications. 2015. Available: https://wwwfdagov/drugs/drug-safety-and-availability/fda-revises-labels-...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
