The proton channel OTOP1 is a sensor for the taste of ammonium chloride
- PMID: 37798269
- PMCID: PMC10556057
- DOI: 10.1038/s41467-023-41637-4
The proton channel OTOP1 is a sensor for the taste of ammonium chloride
Abstract
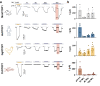
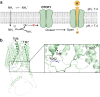
Ammonium (NH4+), a breakdown product of amino acids that can be toxic at high levels, is detected by taste systems of organisms ranging from C. elegans to humans and has been used for decades in vertebrate taste research. Here we report that OTOP1, a proton-selective ion channel expressed in sour (Type III) taste receptor cells (TRCs), functions as sensor for ammonium chloride (NH4Cl). Extracellular NH4Cl evoked large dose-dependent inward currents in HEK-293 cells expressing murine OTOP1 (mOTOP1), human OTOP1 and other species variants of OTOP1, that correlated with its ability to alkalinize the cell cytosol. Mutation of a conserved intracellular arginine residue (R292) in the mOTOP1 tm 6-tm 7 linker specifically decreased responses to NH4Cl relative to acid stimuli. Taste responses to NH4Cl measured from isolated Type III TRCs, or gustatory nerves were strongly attenuated or eliminated in an Otop1-/- mouse strain. Behavioral aversion of mice to NH4Cl, reduced in Skn-1a-/- mice lacking Type II TRCs, was entirely abolished in a double knockout with Otop1. These data together reveal an unexpected role for the proton channel OTOP1 in mediating a major component of the taste of NH4Cl and a previously undescribed channel activation mechanism.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Auron A, Brophy PD. Hyperammonemia in review: pathophysiology, diagnosis, and treatment. Pediatr. Nephrol. 2012;27:207–222. - PubMed
-
- Batshaw ML. Hyperammonemia. Curr. Probl. Pediatr. 1984;14:1–69. - PubMed
-
- van der Klaauw NJ, Smith DV. Taste quality profiles for fifteen organic and inorganic salts. Physiol. Behav. 1995;58:295–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

