COVID-19 mRNA vaccine-mediated antibodies in human breast milk and their association with breast milk microbiota composition
- PMID: 37798293
- PMCID: PMC10556030
- DOI: 10.1038/s41541-023-00745-4
COVID-19 mRNA vaccine-mediated antibodies in human breast milk and their association with breast milk microbiota composition
Abstract
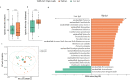
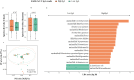
Newborns can acquire immunological protection to SARS-CoV-2 through vaccine-conferred antibodies in human breast milk. However, there are some concerns around lactating mothers with regards to potential short- and long-term adverse events and vaccine-induced changes to their breast milk microbiome composition, which helps shape the early-life microbiome. Thus, we sought to explore if SARS-CoV-2 mRNA vaccine could change breast milk microbiota and how the changes impact the levels of antibodies in breast milk. We recruited 49 lactating mothers from Hong Kong who received two doses of BNT162b2 vaccine between June 2021 and August 2021. Breast milk samples were self-collected by participants pre-vaccination, one week post-first dose, one week post-second dose, and one month post-second dose. The levels of SARS-CoV-2 spike-specific IgA and IgG in breast milk peaked at one week post-second dose. Subsequently, the levels of both antibodies rapidly waned in breast milk, with IgA levels returning to baseline levels one month post-second dose. The richness and composition of human breast milk microbiota changed dynamically throughout the vaccination regimen, but the abundances of beneficial microbes such as Bifidobacterium species did not significantly change after vaccination. Additionally, we found that baseline breast milk bacterial composition can predict spike-specific IgA levels at one week post-second dose (Area Under Curve: 0.72, 95% confidence interval: 0.58-0.85). Taken together, our results identified specific breast milk microbiota markers associated with high levels of IgA in the breast milk following BNT162b2 vaccine. Furthermore, in lactating mothers, BNT162b2 vaccines did not significantly reduce probiotic species in breast milk.
© 2023. Springer Nature Limited.
Conflict of interest statement
F.K.L.C. is Board Member of CUHK Medical Centre. He is a co-founder, non-executive Board Chairman, non-executive scientific advisor, Chief Medical Officer and shareholder of GenieBiome Ltd. He receives patent royalties through his affiliated institutions. He has received fees as an advisor and honoraria as a speaker for Eisai Co. Ltd., AstraZeneca, Pfizer Inc., Takeda Pharmaceutical Co., and Takeda (China) Holdings Co. Ltd. S.C.N. has served as an advisory board member for Pfizer, Ferring, Janssen, and Abbvie and received honoraria as a speaker for Ferring, Tillotts, Menarini, Janssen, Abbvie, and Takeda. S.C.N. has received research grants through her affiliated institutions from Olympus, Ferring, and Abbvie. S.C.N. is a founder member, non-executive director, non-executive scientific advisor, and shareholder of GenieBiome Ltd. S.C.N. and H.M.T. receive patent royalties through her affiliated institutions. F.K.L.C., S.C.N., H.M.T. are named inventors of patent applications held by the CUHK and MagIC that cover the therapeutic and diagnostic use of microbiome. Other authors declare no financial or non-financial competing interests.
Figures



References
-
- Medicine, A. O. B. Considerations for COVID-19 Vaccination in Lactation, https://www.bfmed.org/abm-statement-considerations-for-covid-19-vaccinat... Accessed Feburary 16, 2023 (2020).
-
- Gynecologists, T. A. C. O. O. A. COVID-19 Vaccination Considerations for Obstetric–Gynecologic Care. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articl... Accessed Feburary 16, 2023 (2023).
-
- Prevention, C. F. D. C. A. COVID-19 Vaccines While Pregnant or Breastfeeding, https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregn... Accessed Feburary 16, 2023 (2022).
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

