Muscimol inhibits plasma membrane rupture and ninjurin-1 oligomerization during pyroptosis
- PMID: 37798443
- PMCID: PMC10556065
- DOI: 10.1038/s42003-023-05354-4
Muscimol inhibits plasma membrane rupture and ninjurin-1 oligomerization during pyroptosis
Erratum in
-
Author Correction: Muscimol inhibits plasma membrane rupture and ninjurin-1(NINJ1) oligomerization during pyroptosis.Commun Biol. 2024 Mar 11;7(1):310. doi: 10.1038/s42003-024-05975-3. Commun Biol. 2024. PMID: 38467884 Free PMC article. No abstract available.
Abstract
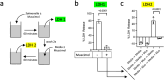
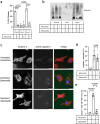
Pyroptosis is a cell death process that causes inflammation and contributes to numerous diseases. Pyroptosis is mediated by caspase-1 family proteases that cleave the pore-forming protein gasdermin D, causing plasma membrane rupture and release of pathogenic cellular contents. We previously identified muscimol as a small molecule that prevents plasma membrane rupture during pyroptosis via an unidentified mechanism. Here, we show that muscimol has reversible activity to prevent cellular lysis without affecting earlier pyroptotic events. Although muscimol is a well-characterized agonist for neuronal GABAA receptors, muscimol protection is not altered by GABAA receptor antagonists or recapitulated by other GABAA agonists, suggesting that muscimol acts via a novel mechanism. We find that muscimol blocks oligomerization of ninjurin-1, which is required for plasma membrane rupture downstream of gasdermin D pore formation. Our structure-activity relationship studies reveal distinct molecular determinants defining inhibition of pyroptotic lysis compared to GABAA binding. In addition, we demonstrate that muscimol reduces lethality during LPS-induced septic shock. Together, these findings demonstrate that ninjurin-1-mediated plasma membrane rupture can be pharmacologically modulated and pave the way toward identification of therapeutic strategies for pathologic conditions associated with pyroptosis.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

