Efficacy and Safety of Reparixin in Patients with Severe COVID-19 Pneumonia: A Phase 3, Randomized, Double-Blind Placebo-Controlled Study
- PMID: 37798468
- PMCID: PMC10600076
- DOI: 10.1007/s40121-023-00871-5
Efficacy and Safety of Reparixin in Patients with Severe COVID-19 Pneumonia: A Phase 3, Randomized, Double-Blind Placebo-Controlled Study
Abstract
Introduction: Polymorphonuclear cell influx into the interstitial and bronchoalveolar spaces is a cardinal feature of severe coronavirus disease 2019 (COVID-19), principally mediated by interleukin-8 (IL-8). We sought to determine whether reparixin, a novel IL-8 pathway inhibitor, could reduce disease progression in patients hospitalized with severe COVID-19 pneumonia.
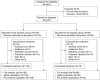
Methods: In this Phase 3, randomized, double-blind, placebo-controlled, multicenter study, hospitalized adult patients with severe COVID-19 pneumonia were randomized 2:1 to receive oral reparixin 1200 mg three times daily or placebo for up to 21 days or until hospital discharge. The primary endpoint was the proportion of patients alive and free of respiratory failure at Day 28, with key secondary endpoints being the proportion of patients free of respiratory failure at Day 60, incidence of intensive care unit (ICU) admission by Day 28 and time to recovery by Day 28.
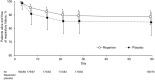
Results: Of 279 patients randomized, 182 received at least one dose of reparixin and 88 received placebo. The proportion of patients alive and free of respiratory failure at Day 28 was similar in the two groups {83.5% versus 80.7%; odds ratio 1.63 [95% confidence interval (CI) 0.75, 3.51]; p = 0.216}. There were no statistically significant differences in the key secondary endpoints, but a numerically higher proportion of patients in the reparixin group were alive and free of respiratory failure at Day 60 (88.7% versus 84.6%; p = 0.195), fewer required ICU admissions by Day 28 (15.8% versus 21.7%; p = 0.168), and a higher proportion recovered by Day 28 compared with placebo (81.6% versus 74.9%; p = 0.167). Fewer patients experienced adverse events with reparixin than placebo (45.6% versus 54.5%), most mild or moderate intensity and not related to study treatment.
Conclusions: This trial did not meet the primary efficacy endpoints, yet reparixin showed a trend toward limiting disease progression as an add-on therapy in COVID-19 severe pneumonia and was well tolerated.
Trial registration: ClinicalTrials.gov: NCT04878055, EudraCT: 2020-005919-51.
Keywords: COVID-19; Interleukin-8 (IL-8); Reparixin; SARS-CoV-2.
© 2023. The Author(s).
Conflict of interest statement
Lorenzo Piemonti has no other conflicts of interest to disclose. Giovanni Landoni has no other conflicts of interest to disclose. Antonio Voza declares consulting fees from Sildeha Pharma Swisse; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Minerva Medica and Viatris; payment for expert testimony from GlaxoSmithKline, Alfa Sigma (to his institution), and Aurora Biopharma; and unpaid roles as guest editor of special issue on COVID-19: Prognosis and Long-term Sequelae’ on Viruses, and an advisory board for Critical Reviews in Oncology/Hematology, all outside the scope of the current manuscript. Massimo Puoti declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Gilead Sciences, GlaxoSmithKline, and AstraZeneca, all outside the scope of the current manuscript. Ivan Gentile declares departmental grants from Gilead; personal fees and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from MSD, AbbVie, Gilead, Pfizer, GlaxoSmithKline, SOBI, Nordic/InfectoPharm, Angelini, Abbott, and Basilea; support for attending a meeting from Janssen and Gilead; and participation on a data safety monitoring board or advisory board for MSD, AbbVie, Gilead, Pfizer, GlaxoSmithKline, SOBI, Nordic/InfectoPharm, Angelini, Abbott, and Basilea, all outside the scope of the current manuscript. Nicola Coppola has no other conflicts of interest to disclose. Stefano Nava has no other conflicts of interest to disclose. Alessia Mattei has no other conflicts of interest to disclose. Franco Marinangeli declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Grunenthal, Mundipharma, and MSD, all outside the scope of the current manuscript. Giulia Marchetti declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Gilead, ViiV, MSD, Shionogi, and Angelini; support for attending meetings and/or travel from Jannsen and ViiV; and participation on a data safety monitoring board or advisory board for Viiv and Gilead, all outside the scope of the current manuscript. Paolo Bonfanti declares consulting fees and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Viiv, Gilead, Merck, Jannsen, and Pfizer, all outside the scope of the current manuscript. Claudio Maria Mastroianni declares consulting fees from Gilead and VIIV; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from MSD, VIIV, Gilead, Menarini, AstraZeneca, and Pfizer; and support for attending meetings and/or travel from Menarini, all outside the scope of the current manuscript. Matteo Bassetti declares payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Angelini, Astellas, Bayer, Biomerieux, Cidara, Gilead, Menarini, MSD, Nabriva, and Shionogi, and participation on a data safety monitoring board or advisory board for Angelini, Astellas, Bayer, Biomerieux, Cidara, Gilead, Menarini, MSD, Nabriva, and Shionogi, all outside the scope of the current manuscript. Ernesto Crisafulli declares a grant from GlaxoSmithKline; honoraria for lecturing for AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini, Qbgroup, and Sanofi; honoraria for scientific advisory boards for Chiesi and GlaxoSmithKline; and support for attending meetings and/or travel from Chiesi and GlaxoSmithKline, all outside the scope of the current manuscript. Paolo Antonio Grossi declares consulting fees from Takeda, MSD, and Gilead; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Biotest and Gilead; and participation on a data safety monitoring board or advisory board for Reithera, all outside the scope of the current manuscript. Alberto Zangrillo has no other conflicts of interest to disclose. Antonio Desai has no other conflicts of interest to disclose. Marco Merli declares grants or contracts from Gilead, and support for attending meetings and/or travel from Pfizer, all outside the scope of the current manuscript. Maria Foggia has no other conflicts of interest to disclose. Marco Carpano has no other conflicts of interest to disclose. Lorenzo Schiavoni has no other conflicts of interest to disclose. Antonella D’Arminio Monforte has no other conflicts of interest to disclose. Luca Bisi has no other conflicts of interest to disclose. Gianluca Russo declares support for attending a meeting from Gilead, outside the scope of the current manuscript. Fabiana Busti has no other conflicts of interest to disclose. Cristina Rovelli has no other conflicts of interest to disclose. Elisabetta Perrotta, Giovanni Goisis, Elizabeth M. Gavioli, Sophie Toya, Maria De Pizzol, Flavio Mantelli, Marcello Allegretti, and Enrico Maria Minnella are employees of Dompé Farmaceutici SpA, the sponsor of the study.
Figures
Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
A Multicenter Phase 2 Randomized Controlled Study on the Efficacy and Safety of Reparixin in the Treatment of Hospitalized Patients with COVID-19 Pneumonia.Infect Dis Ther. 2022 Aug;11(4):1559-1574. doi: 10.1007/s40121-022-00644-6. Epub 2022 May 26. Infect Dis Ther. 2022. PMID: 35618953 Free PMC article.
-
Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 11;22(1):42. doi: 10.1186/s13063-020-04998-5. Trials. 2021. PMID: 33430924 Free PMC article.
-
A Phase I/II Clinical Trial to evaluate the efficacy of baricitinib to prevent respiratory insufficiency progression in onco-hematological patients affected with COVID19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Feb 5;22(1):116. doi: 10.1186/s13063-021-05072-4. Trials. 2021. PMID: 33546739 Free PMC article.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
Cited by
-
A narrative review of chemokine receptors CXCR1 and CXCR2 and their role in acute respiratory distress syndrome.Eur Respir Rev. 2024 Jul 24;33(173):230172. doi: 10.1183/16000617.0172-2023. Print 2024 Jul. Eur Respir Rev. 2024. PMID: 39048127 Free PMC article. Review.
-
Preliminary results and a theoretical perspective of co‑treatment using a miR‑93‑5p mimic and aged garlic extract to inhibit the expression of the pro‑inflammatory interleukin‑8 gene.Exp Ther Med. 2025 Feb 25;29(4):85. doi: 10.3892/etm.2025.12835. eCollection 2025 Apr. Exp Ther Med. 2025. PMID: 40084194 Free PMC article.
-
Targeting CXCL8 signaling sensitizes HNSCC to anlotinib by reducing tumor-associated macrophage-derived CLU.J Exp Clin Cancer Res. 2025 Feb 5;44(1):39. doi: 10.1186/s13046-025-03298-7. J Exp Clin Cancer Res. 2025. PMID: 39905539 Free PMC article.
References
-
- Masso-Silva JA, Moshensky A, Lam MTY, Odish MF, Patel A, Xu L, et al. Increased peripheral blood neutrophil activation phenotypes and neutrophil extracellular trap formation in critically ill coronavirus disease 2019 (COVID-19) patients: a case series and review of the literature. Clin Infect Dis. 2022;74:479–489. doi: 10.1093/cid/ciab437. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

