Spatial heterogeneity of bone marrow endothelial cells unveils a distinct subtype in the epiphysis
- PMID: 37798545
- PMCID: PMC10567563
- DOI: 10.1038/s41556-023-01240-7
Spatial heterogeneity of bone marrow endothelial cells unveils a distinct subtype in the epiphysis
Abstract
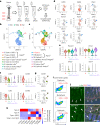
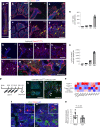
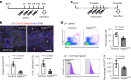
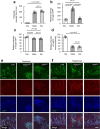
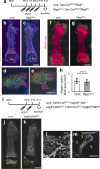
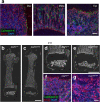
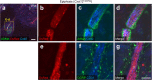
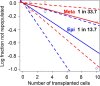
Bone marrow endothelial cells (BMECs) play a key role in bone formation and haematopoiesis. Although recent studies uncovered the cellular taxonomy of stromal compartments in the bone marrow (BM), the complexity of BMECs is not fully characterized. In the present study, using single-cell RNA sequencing, we defined a spatial heterogeneity of BMECs and identified a capillary subtype, termed type S (secondary ossification) endothelial cells (ECs), exclusively existing in the epiphysis. Type S ECs possessed unique phenotypic characteristics in terms of structure, plasticity and gene expression profiles. Genetic experiments showed that type S ECs atypically contributed to the acquisition of bone strength by secreting type I collagen, the most abundant bone matrix component. Moreover, these cells formed a distinct reservoir for haematopoietic stem cells. These findings provide the landscape for the cellular architecture in the BM vasculature and underscore the importance of epiphyseal ECs during bone and haematopoietic development.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. - PubMed
-
- Augustin HG, Koh GY. Organotypic vasculature: from descriptive heterogeneity to functional pathophysiology. Science. 2017;357:eaal2379. - PubMed
-
- Kalucka J, et al. Single-cell transcriptome atlas of murine endothelial cells. Cell. 2020;180:764–779.e720. - PubMed
-
- Vanlandewijck M, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–480. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

