Genetic Inhibition of APOL1 Pore-Forming Function Prevents APOL1-Mediated Kidney Disease
- PMID: 37798822
- PMCID: PMC10631602
- DOI: 10.1681/ASN.0000000000000219
Genetic Inhibition of APOL1 Pore-Forming Function Prevents APOL1-Mediated Kidney Disease
Abstract
Significance statement: African Americans are at increased risk of CKD in part due to high-risk (HR) variants in the apolipoprotein L1 ( APOL1 ) gene, termed G1/G2. A different APOL1 variant, p.N264K , reduced the risk of CKD and ESKD among carriers of APOL1 HR variants to levels comparable with individuals with APOL1 low-risk variants in an analysis of 121,492 participants of African ancestry from the Million Veteran Program (MVP). Functional genetic studies in cell models showed that APOL1 p.N264K blocked APOL1 pore-forming function and ion channel conduction and reduced toxicity of APOL1 HR mutations. Pharmacologic inhibitors that mimic this mutation blocking APOL1 -mediated pore formation may be able to prevent and/or treat APOL1 -associated kidney disease.
Background: African Americans are at increased risk for nondiabetic CKD in part due to HR variants in the APOL1 gene.
Methods: We tested whether a different APOL1 variant, p.N264K , modified the association between APOL1 HR genotypes (two copies of G1/G2) and CKD in a cross-sectional analysis of 121,492 participants of African ancestry from the MVP. We replicated our findings in the Vanderbilt University Biobank ( n =14,386) and National Institutes of Health All of Us ( n =14,704). Primary outcome was CKD and secondary outcome was ESKD among nondiabetic patients. Primary analysis compared APOL1 HR genotypes with and without p.N264K . Secondary analyses included APOL1 low-risk genotypes and tested for interaction. In MVP, we performed sequential logistic regression models adjusting for demographics, comorbidities, medications, and ten principal components of ancestry. Functional genomic studies expressed APOL1 HR variants with and without APOL1 p.N264K in cell models.
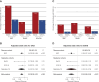
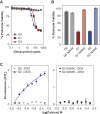
Results: In the MVP cohort, 15,604 (12.8%) had two APOL1 HR variants, of which 582 (0.5%) also had APOL1 p.N264K . In MVP, 18,831 (15%) had CKD, 4177 (3%) had ESKD, and 34% had diabetes. MVP APOL1 HR, without p.N264K , was associated with increased odds of CKD (odds ratio [OR], 1.72; 95% confidence interval [CI], 1.60 to 1.85) and ESKD (OR, 3.94; 95% CI, 3.52 to 4.41). In MVP, APOL1 p.N264K mitigated the renal risk of APOL1 HR, in CKD (OR, 0.43; 95% CI, 0.28 to 0.65) and ESKD (OR, 0.19; CI 0.07 to 0.51). In the replication cohorts meta-analysis, APOL1 p.N264K mitigated the renal risk of APOL1 HR in CKD (OR, 0.40; 95% CI, 0.18 to 0.92) and ESKD (OR, 0.19; 95% CI, 0.05 to 0.79). In the mechanistic studies, APOL1 p.N264K blocked APOL1 pore-forming function and ion channel conduction and reduced toxicity of APOL1 HR variants.
Conclusions: APOL1 p.N264K is associated with reduced risk of CKD and ESKD among carriers of APOL1 HR to levels comparable with individuals with APOL1 low-risk genotypes.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Society of Nephrology.
Conflict of interest statement
V.A. Assimon reports Employer: Cartography Biosciences, Maze Therapeutics, and Soteria Biotherapeutics; Consultancy: Capstan Therapeutics and Soteria Biotherapeutics; Ownership Interest: Cartography Biosciences, Cleave Therapeutics, Denali Therapeutics, Maze Therapeutics, and Soteria Biotherapeutics; Research Funding: Cartography Biosciences, Maze Therapeutics, and Soteria Biotherapeutics; and Patents or Royalties: Maze Therapeutics, Soteria Biotherapeutics, and University of California San Francisco. A.G. Bick reports Ownership Interest: TenSixteen Bio. H. Chan reports Employer: Maze Therapeutics; and Ownership Interest: Cytokinetics, Gilead, Illumina, Nektar, and Vertex.S. Chandrasekar reports Employer: Apple Inc., Arcus Biosciences, and Maze Therapeutics; and Ownership Interest: Apple Inc., Arcus Biosciences, and Maze Therapeutics. C.P. Chung reports Advisory or Leadership Role: Journals:
Because Katalin Susztak is an editor of the
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

