Combining Glucose Monitoring and Insulin Infusion in an Integrated Device: A Narrative Review of Challenges and Proposed Solutions
- PMID: 37798963
- PMCID: PMC11874455
- DOI: 10.1177/19322968231203237
Combining Glucose Monitoring and Insulin Infusion in an Integrated Device: A Narrative Review of Challenges and Proposed Solutions
Abstract
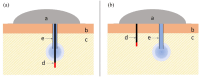
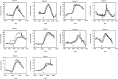
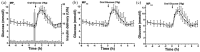
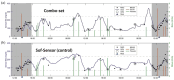
The introduction of automated insulin delivery (AID) systems has enabled increasing numbers of individuals with type 1 diabetes (T1D) to improve their glycemic control largely. However, use of AID systems is limited due to their complexity and costs associated. The user must wear both a continuously monitoring glucose system and an insulin infusion pump. The glucose sensor and the insulin catheter must be inserted at two different body sites using different insertion devices. In addition, the user must pair and manage the different systems. These communicate with the AID software implemented on the pump or on a third device such as a dedicated display device or smart phone application. These components might be developed and commercialized by different manufacturers, which in turn can cause difficulties for patients seeking technical support. A possible solution to these challenges would be to integrate the glucose sensor and insulin catheter into a single device. This would allow the glucose sensor and insulin catheter to be inserted simultaneously, eliminating the need for pairing, and simplifying system management. In recent years, different technologies have been developed and evaluated in clinical investigations that combine the glucose sensor and the insulin catheter in one platform. The consistent finding of all these studies is that integration has no adverse effect on insulin infusion and glucose measurements provided that certain conditions are met. In this review, we discuss the perceived challenges of such an approach and discuss possible solutions that have been proposed.
Keywords: accuracy; automated insulin delivery; colocalization; continuous glucose monitoring; insulin delivery; integration.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MS and AM are full-time employees of PharmaSens AG; JKM is a member of advisory boards of Abbott Diabetes Care, Becton-Dickinson, Boehringer Ingelheim, Eli Lilly, Embecta, Medtronic, NovoNordisk A/S, Roche Diabetes Care, Sanofi-Aventis, Viatris and received speaker honoraria from A. Menarini Diagnostics, Abbott Diabetes Care, AstraZeneca, Boehringer Ingelheim, Dexcom, Eli Lilly, Medtrust, MSD, NovoNordisk A/S, Roche Diabetes Care, Sanofi, Servier, and Ypsomed; she is shareholder of decide Clinical Software GmbH and elyte Diagnostics where she also serves as CMO; GF has received speakers honoraria or consulting fees from Abbott, Ascensia, Berlin Chemie, Boydsense, Dexcom, Lilly, Metronom, MySugr, Novo Nordisk, PharmaSens, Roche, Sanofi, and Terumo; P-YB has received speaker honoraria from Abbott, Eli Lilly, Novo Nordisk, and Sanofi, is chief medical officer for Diabeloop, and served on advisory board panels for Abbott, Dexcom, Insulet, LifeScan, Eli Lilly, Pharmasens, Novo Nordisk, and Sanofi; Peter Diem is member of the PharmaSens AG Board of Directors; Lutz Heinemann is a consultant to several diagnostic and therapeutic companies in the development of new products, he is a shareholder in the Profil Institute for Metabolic Research in Neuss, Germany.
Figures
References
-
- McAuley SA, Lee MH, Paldus B, et al. Six months of hybrid closed-loop versus manual insulin delivery with fingerprick blood glucose monitoring in adults with type 1 diabetes: a randomized, controlled trial. Diabetes Care. 2020;43(12):3024-3033. - PubMed
-
- Collyns OJ, Meier RA, Betts ZL, et al. Improved glycemic outcomes with Medtronic MiniMed advanced hybrid closed-loop delivery: results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose suspend in people with type 1 diabetes. Diabetes Care. 2021;44(4):969-975. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

