Self-assembled adipose-derived mesenchymal stem cells as an extracellular matrix component- and growth factor-enriched filler
- PMID: 37799276
- PMCID: PMC10549996
- DOI: 10.3389/fcell.2023.1219739
Self-assembled adipose-derived mesenchymal stem cells as an extracellular matrix component- and growth factor-enriched filler
Abstract
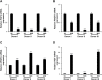
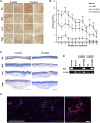
The clinical application of mesenchymal stem cells (MSCs) is attracting attention due to their excellent safety, convenient acquisition, multipotency, and trophic activity. The clinical effectiveness of transplanted MSCs is well-known in regenerative and immunomodulatory medicine, but there is a demand for their improved viability and regenerative function after transplantation. In this study, we isolated MSCs from adipose tissue from three human donors and generated uniformly sized MSC spheroids (∼100 µm in diameter) called microblocks (MiBs) for dermal reconstitution. The viability and MSC marker expression of MSCs in MiBs were similar to those of monolayer MSCs. Compared with monolayer MSCs, MiBs produced more extracellular matrix (ECM) components, including type I collagen, fibronectin, and hyaluronic acid, and growth factors such as vascular endothelial growth factor and hepatocyte growth factor. Subcutaneously injected MiBs showed skin volume retaining capacity in mice. These results indicate that MiBs could be applied as regenerative medicine for skin conditions such as atrophic scar by having high ECM and bioactive factor expression.
Keywords: atrophic scar in addition; extracellular matrix; growth factor; mesenchymal stem cell; microblock; regenerative medicine; spheroid.
Copyright © 2023 Park, Lee, Park, Yoo, Kwon, Park, Kang, Lee and Cho.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Long-Duration Three-Dimensional Spheroid Culture Promotes Angiogenic Activities of Adipose-Derived Mesenchymal Stem Cells.Biomol Ther (Seoul). 2016 May 1;24(3):260-7. doi: 10.4062/biomolther.2015.146. Biomol Ther (Seoul). 2016. PMID: 26869524 Free PMC article.
-
Current regenerative medicine-based approaches for skin regeneration: A review of literature and a report on clinical applications in Japan.Regen Ther. 2022 Jun 15;21:73-80. doi: 10.1016/j.reth.2022.05.008. eCollection 2022 Dec. Regen Ther. 2022. PMID: 35785041 Free PMC article. Review.
-
Regenerative medicine strategies for hair growth and regeneration: A narrative review of literature.Regen Ther. 2022 Oct 31;21:527-539. doi: 10.1016/j.reth.2022.10.005. eCollection 2022 Dec. Regen Ther. 2022. PMID: 36382136 Free PMC article. Review.
-
Mesenchymal Stem Cell Spheroids Retain Osteogenic Phenotype Through α2β1 Signaling.Stem Cells Transl Med. 2016 Sep;5(9):1229-37. doi: 10.5966/sctm.2015-0412. Epub 2016 Jun 30. Stem Cells Transl Med. 2016. PMID: 27365484 Free PMC article.
-
Human Adipose-Derived Mesenchymal Stromal/Stem Cell Spheroids Possess High Adipogenic Capacity and Acquire an Adipose Tissue-like Extracellular Matrix Pattern.Tissue Eng Part A. 2020 Aug;26(15-16):915-926. doi: 10.1089/ten.TEA.2019.0206. Epub 2020 Apr 9. Tissue Eng Part A. 2020. PMID: 32070231
References
-
- Cheng L.-Y., Sun X.-M., Tang M.-Y., Jin R., Cui W.-G., Zhang Y.-G. (2016). An update review on recent skin fillers. Plast. Aesthet. Res. 3, 92–99. 10.20517/2347-9264.2015.124 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

