Clinical Implications and Dynamics of Clonal Hematopoiesis in Anti-CD19 CAR T-cell Treated Patients
- PMID: 37799345
- PMCID: PMC10550045
- DOI: 10.1097/HS9.0000000000000957
Clinical Implications and Dynamics of Clonal Hematopoiesis in Anti-CD19 CAR T-cell Treated Patients
Abstract
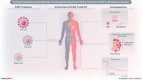
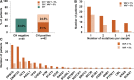
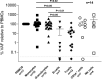
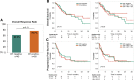
Recent evidence revealed important interactions between clonal hematopoiesis (CH) and cellular therapies established for the treatment of hematologic malignancies. The impact of CH on safety, efficacy, and outcome of chimeric antigen receptor (CAR) T-cell therapy is currently under investigation. We analyzed 110 patients with relapsed/refractory B-cell non-Hodgkin lymphoma (n = 105) or acute lymphoblastic leukemia (ALL) (n = 5), treated with Axicabtagene-Ciloleucel (39%), Tisagenlecleucel (51%), or Brexucabtagene autoleucel (10%). Using error-corrected targeted sequencing, a high CH prevalence of 56.4% (variant allele frequency [VAF] ≥1%) at the time of CAR T-cell infusion was detected. The most frequently mutated gene was PPM1D followed by DNMT3A, TET2, ASXL1, and TP53. Variant allele frequencies were significantly lower in B and T cells compared with monocytes and granulocytes. CH did not increase the risk of CAR T-related toxicities. The incidences of cytokine release syndrome and immune effector-cell-associated neurotoxicity syndrome were similar between CHpos and CHneg patients, regardless of clone size, age, or CAR T product. Prolonged cytopenias were not associated with CH. Best overall response rates (ORRs) were numerically but not significantly higher in CHpos patients (ORR 76.7% versus 62.2%; P = 0.13). Furthermore, CH status did not predict progression-free survival or overall survival. Lastly, sequential analysis showed a modest VAF increase of 1.3% and acquisition of novel mutations within 100 days postinfusion. CH was frequent in large B-cell lymphoma/ALL patients receiving CAR T-cells but did not affect toxicity nor treatment response or outcome.
Copyright © 2023 the Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the European Hematology Association.
Conflict of interest statement
MH reports fees for advisory or consultancy services from Abbvie, Agios, BMS, Daiichi Sankyo, Eurocept, Glycostem, Janssen, Jazz Pharmaceuticals, Kura Oncology, Novartis, Pfizer, PinotBio, Roche, Takeda, and Tolremo. FD reports personal fees from Gilead, Incyte, Roche, Novartis, AbbVie, Astra Zeneca outside the submitted work. JFK reports personal fees from Gilead and Janssen outside the submitted work. GW reports honoraria from Gilead, Novartis, Clinigen, and Janssen outside the submitted work. OP has no conflicts of interest directly related to this work. OP has received honoraria or travel support from Gilead, Jazz, MSD, Novartis, Pfizer, and Therakos. He has received research support from Incyte and Priothera. He is member of advisory boards to Equillium Bio, Jazz, Gilead, Novartis, MSD, Omeros, Priothera, Sanofi, Shionogi, and SOBI. All the other authors have no conflicts of interest to disclose.
Figures


References
-
- Buscarlet M, Provost S, Zada YF, et al. DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood. 2017;130:753–762. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
